Food and Dietary Supplement Labeling: What Comes Next?
Labeling of food and dietary supplements in the United States involve several aspects and each must be approached with careful consideration. Regulatory, scientific, and business decisions need to be considered when working on labels’ mandatory elements and claims. The FDA released in December 2024 and January 2025 a few new proposed and final rules on several issues that will impact food and dietary supplement labeling. 1,2,3,4 However, it is unclear at this time whether these rules will be approved by the new federal administration. 5 Stay tuned into these topics over the next few months to obtain information on the final requirements.
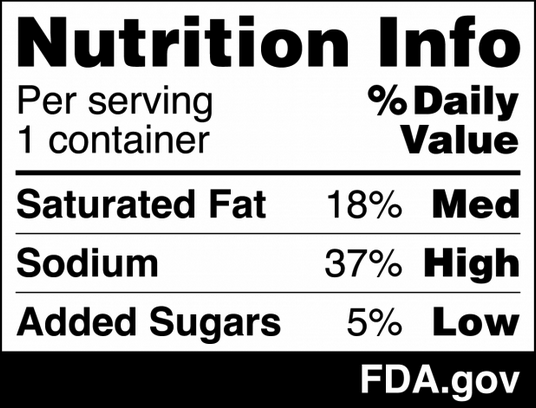
Mandatory elements of the label declared on the principal display panel (or front-of-package) include the statement of identity and net content of the product. Other elements such as warnings might also be required depending on the product. One of the newly proposed rules by the FDA is the inclusion of an abbreviated version of the nutrition information (“Nutrition Info Box”) to be placed on the front of the package of most packaged foods. 1 If approved, companies will have 3 to 4 years (depending on business size) to implement the new label. Although editing a label is not very challenging, some companies might decide to reformulate the entire product in order to avoid a potential drop in sales. The nutrition info box will include clear information of product’s level as low, medium, or high of saturated fats, sodium, and added sugars on the front-of-package. In addition, the FDA released a draft guidance (nonbinding recommendations) on the statement of identity of plant-based foods. Coming up with the statement of identity of non-standardized foods can be tricky. This FDA guidance provides examples of how to name plant-based foods such as “soy-based cheddar cheese,” “chickpea and lentil-based fish sticks,” and “chia and flaxseed egg-less scramble.” This draft guidance is open to receive comments. The period for comments ends on May 7, 2025, and can be submitted online. 2
The other mandatory elements of the label are declared in the information panel. This is the panel located immediately to the right of the principal display panel and includes the Nutrition/Supplement Facts panel, the list of other ingredients (including allergen declaration), and the name and place of business. One of the FDA’s newly proposed rules that will impact the information panel if approved is the ban on Red No. 3 color additive. 3 Prohibiting the use FD&C Red No.3 will cause companies to reformulate their products. Food companies will have until January 15, 2027 to comply. Imported foods will also have to comply. This rule came into decision due to data that Red No.3 dye might induce cancer. The FDA also released a final guidance for industry regarding food allergen labeling. This guidance provides several new questions and answers on allergens such as shellfish and fish species as well as on allergen-free claims. Although this is a final guidance, comments can be submitted any time. 4
Claims are another important piece of the labeling. Claims inform consumers of the intended use of the product as well as add product marketability. However, as with the other components of the label, ensuring compliance with the correct claims wording and claims substantiation will keep companies out of trouble. The new FDA rule that will affect product claims if approved is on the term “healthy.” In December 2024, the FDA released a final rule on the eligibility for use of the term “healthy” as it relates to nutrient content claims in food and dietary supplements. This rule is set to be effective on February 25, 2025 with compliance until February 25, 2028. However, again it is unclear if this final rule will remain after the new federal administration. In general, the term “healthy” will only be allowed when products meet the criteria of containing a certain amount of healthy nutrients (i.e., fruits, vegetables, grains, fat-free and low-fat dairy and proteins) as well as staying under the limit for unhealthy nutrients including added sugars, saturated fat and sodium. 5
References
- FDA Proposes Requiring At-a-Glance Nutrition Information on the Front of Packaged Foods. Content current as of: 01/14/2025. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-proposes-requiring-glance-nutrition-information-front-packaged-foods
- S. Department of Health and Human Services – Food and Drug Administration – Human Foods Program. Labeling of Plant-Based Alternatives to Animal-Derived Foods: Draft Guidance for Industry – Draft Guidance. January 2025. Retrieved from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/draft-guidance-industry-labeling-plant-based-alternatives-animal-derived-foods
- FDA to Revoke Authorization for the Use of Red No. 3 in Food and Ingested Drugs. January 15, 2025. Retrieved from https://www.fda.gov/food/hfp-constituent-updates/fda-revoke-authorization-use-red-no-3-food-and-ingested-drugs?utm_medium=email&utm_source=govdelivery
- Guidance for Industry: Questions and Answers Regarding Food Allergen Labeling (Edition 5). January 2025. Retrieved from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-questions-and-answers-regarding-food-allergen-labeling-edition-5
- Federal Register. Food Labeling: Nutrient Content Claims; Definitions of Term “Healthy”. Final Rule. 12/27/2024. Retrieved from https://www.federalregister.gov/documents/2024/12/27/2024-29957/food-labeling-nutrient-content-claims-definition-of-term-healthy
- U.S. Department of Health and Human Services – Food and Drug Administration – Use of the term healthy on food labeling. Current as of: 01/16/2025. Retrieved from: https://www.fda.gov/food/nutrition-food-labeling-and-critical-foods/use-term-healthy-food-labeling
- White House – Presidential Actions. Regulatory Freeze Pending Review. January 20, 2025. Retrieved from https://www.whitehouse.gov/presidential-actions/2025/01/regulatory-freeze-pending-review/?utm_campaign=EAS&utm_medium=email&_hsenc=p2ANqtz-_HHp0nhlpXvYDyI9YOR-vQDb1SlAgnpQqVgmz8ThSBYafnpoq4dU9cxrRdWE-bzXjjg5QSK9cAKqt0pJyAXSmuO2JP-Yjk7A_ym9DL2xL6O49XJRI&_hsmi=343689829&utm_content=343689829&utm_source=hs_email
