Status and Outlook of Food Labeling Proposals
When the National Bioengineered Food Disclosure Standard was signed into law in July 2016, it represented the culmination of an extended legislative and regulatory period of activity on food labeling issues in general. As the focus shifts toward the implementation of these labeling proposals – such as requirements for bioengineered food disclosure, calorie disclosure on menus, and updating the nutrition facts panel – the debate over food labeling issues will remain very active, and new labeling proposals are certain to arise, including ones directly impacting food safety.
Bioengineered Food Disclosure
The National Bioengineered Food Disclosure Standard was enacted into law to preempt a patchwork of differing and conflicting national, state and local requirements that were going into effect or being proposed at the time. The legislation, which acknowledges the proven safety of agricultural biotechnology products while simultaneously ensuring consumers have access to more information about the foods they purchase, was enacted with strong bipartisan support. Because this labeling mandate is based on a desire to enhance consumer information, and not address any safety concerns, Congress delegated responsibility for implementing the disclosure standard to the Agricultural Marketing Service (AMS) within the U.S. Department of Agriculture (USDA).
The disclosure standard, which will be developed by USDA through rulemaking, is intended to apply to a broad category of foods and provide several options for mandatory disclosure, including text on packaging, the use of a symbol, or an electronic or digital link. Additional flexibility will be provided for small food manufacturers and food in very small packages.
Under the law, certain foods are excluded from the disclosure requirement, including food served in restaurants or similar retail food establishments, or food produced by very small food manufacturers and any food that is derived from an animal solely because the animal consumed feed produced from a bioengineered substance. Food products that have meat, poultry or egg as their main ingredient would remain subject to labeling under other federal statutes. The legislation also would ensure that food products certified under the national organic program could display a “non-GMO” label or other similar claim in addition to the USDA organic seal.
Study on Potential Challenges
The law also required USDA to conduct a study to identify any technological or other barriers to disclosure and offer additional disclosure options if the Secretary of Agriculture determined that barriers existed. While the study, which was released in September 2017, did acknowledge some notable challenges, it concluded that most consumers seeking information on their food purchases would be able to access this information, given the proper education and tools.
Challenges identified by the study include: consumers not recognizing the on-package digital link or not associating it with food information; retailers’ unfamiliarity with digital links and inability to assist consumers; some consumer challenges in accessing the tools needed to scan a link; consumers struggling with mobile applications (apps), regardless of comfort level with technology; and broadband connectivity and connection speed.
Although researchers observed these key technological challenges that prevented nearly all participants in the study from obtaining the information through electronic or digital disclosure methods, the study asserted that the challenges could be overcome through appropriate implementation of the law.
Outlook
USDA Secretary Sonny Perdue has expressed his commitment to meeting the implementation deadline of July 29, 2018 for the National Bioengineered Food Disclosure Standard. This summer, the department collected feedback on thirty questions under consideration in connection with the rulemaking process, and released the accessibility study required under the law close to the deadline set by Congress. The fact that consumer groups filed suit to compel the study’s release shortly after the deadline passed serves as an early indicator that all aspects of USDA’s implementation of the law will remain under close scrutiny and, in all likelihood, be the subject of additional litigation.
There also has been some indication that the compliance date will coincide with another labeling requirement in the pipeline – the update to the Nutrition Facts Label.
Update to the Nutrition Facts Label
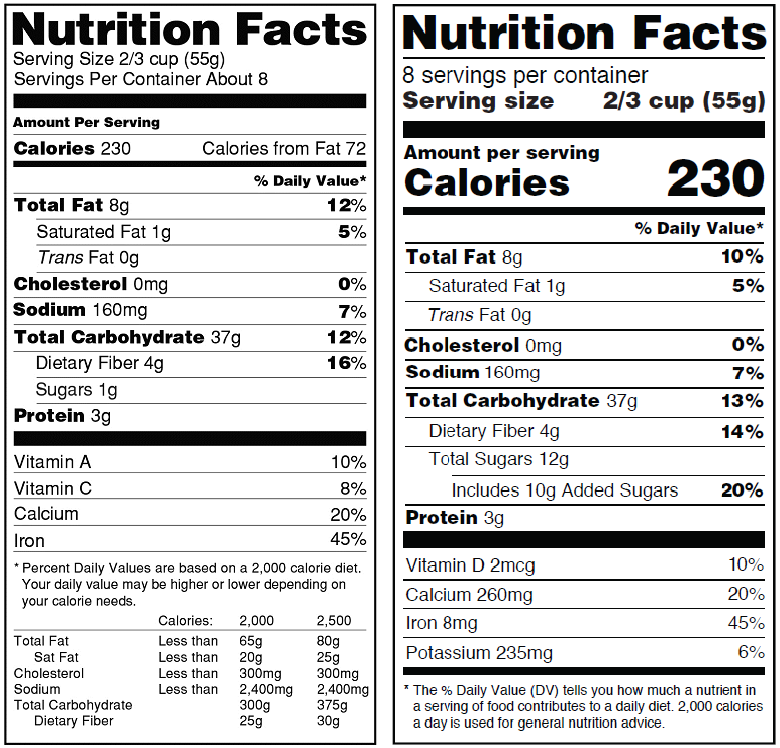
On May 27, 2016, the Food and Drug Administration (FDA) published the final rules updating the Nutrition Facts label for packaged foods to reflect new scientific information, including the link between diet and chronic diseases such as obesity and heart disease. In its announcement, the FDA indicated that the new label would make it easier for consumers to make better-informed food choices.
While the familiar look of the nutrition facts panel remains, FDA is proposing changes that would include an emphasis on Calories by increasing the font size and bolding it. The font size for serving size and servings per container also will be increased.
Other notable changes include requiring manufacturers to declare the actual amount of vitamin D, calcium, iron and potassium, in addition to the percent Daily Value, and to better explain in the footnote what percent Daily Value means. The rule also requires that the label include Added sugars in grams and as percent Daily Value in order to reflect recent scientific data showing that it is difficult to meet nutrient needs while staying within calorie limits if a person consumes more than 10 percent of total daily calories from added sugar.
The rule also requires updates to serving sizes on the nutrition facts label so that they are based on amounts of foods and beverages that people are actually consuming. For example, for packages that are between one and two servings, the rule requires that the calories and other nutrients be labeled as one serving because people typically consume it in one sitting.
Additional details of the update to the Nutrition Facts Label can be found on the FDA web site.
Outlook
On June 13, 2017, FDA announced that it would delay the compliance date for the final rules updating the Nutrition Facts Label, adding that the new date would be forthcoming. In a Federal Register notice on October 2, FDA announced the new compliance date of January 1, 2020.
Earlier in the year, a group of food industry trade associations and officials requested that FDA delay the compliance date for three years, asserting that it should be aligned with the compliance date for USDA’s Bioengineered Food Disclosure Standard. Industry groups had been arguing that aligning these dates would eliminate the need to change labels twice.
Because the Administration has hinted support for aligning the compliance dates for USDA disclosure and FDA labeling requirements, FDA could announce another delay as the January 2020 date approaches.
Continue to page 2 below.
Menu Labeling
Another labeling issue pending before FDA relates to calorie disclosure on restaurant menus. In May 2017, the agency announced that it was delaying the compliance date for menu labeling requirements by a year, from May 5, 2017 to May 7, 2018. FDA explained that the delay would allow industry to identify opportunities to reduce costs and enhance flexibility of the requirements.
In general, convenience stores and grocers supported the delay because of the scope of the food establishments covered under the menu labeling requirements. However, the National Restaurant Association has long supported the rule because it would preempt a number of different requirements on the state and local level.
Frustrated over the delay, two consumer groups – the Center for Science in the Public Interest (CSPI) and National Consumers League – filed a lawsuit claiming that the delay would not assist industry in avoiding compliance costs and therefore, did not represent a good cause for the delay. For its part, FDA filed a motion to dismiss the case, arguing that the consumer groups lacked standing because they had not demonstrated how they were harmed by the delay.
Temporary Truce
FDA and the two groups recently filed a joint motion agreeing to pause litigation on the issue. As part of this agreement, FDA promised to confirm by the end of 2017 the compliance date of May 7, 2018 for the rule, or publish the industry compliance guidance by the end of 2017. If FDA fails to meet these obligations, litigation could resume.
Outlook
This temporary truce likely will prevent state and local governments from proceeding with their own menu labeling efforts, which were preempted by the federal law. Recently, New York City had threatened to begin enforcing its own rules, but reached an agreement with FDA on the May 7, 2018 compliance date. While it was already unlikely that other cities would move forward in light of the NYC-FDA agreement, the temporary truce between the consumer groups and FDA virtually assures it.
Allergens
Undeclared allergens on food labels have been the leading cause of U.S. food recalls for several years now, and it’s a problem that has long vexed the industry, especially given how preventable labeling errors are compared to other threats. According to the Center for Disease Control and Prevention (CDC), over 50 million Americans suffer from food allergies annually.
To address this problem, the Food Marketing Institute (FMI) Foundation recently announced that it is awarding the Food Allergy Research and Resource Program (FARRP) at the University of Nebraska – Lincoln’s Food Science and Technology Department a $20,000 grant to identify root-cause labeling errors. The research also will recommend best-practice procedures for manufacturers, suppliers and retailers in order to reduce undeclared allergen recalls.
In its press release announcing the grant, the FMI Foundation explained that, by focusing on how primary root-cause errors occur and identifying appropriate preventive measures, food allergen recalls can be reduced, thus creating better protection for food allergic consumers, reduce food waste, and decrease economic burden for suppliers, manufacturers and retailers.
The Food Allergy Research and Resource Program intends to present preliminary outcomes of their research at a conference in November 2017, and ultimately submit a paper for peer-reviewed publication.
Outlook
Because this effort is being supported by FMI, a large trade association representing the food retail industry, the recommendations for best-practices that emanate from this research have the potential of being implemented industry-wide on a voluntary basis. Given the likely absence of any legislative or regulatory activity that would be perceived as imposing additional burdens on industry, efforts such as this that are supported by industry and result in voluntary best practice guidelines certainly are worth monitoring.
Simplifying Date Labels
The Consumer Goods Forum, a network of approximately 400 large food and consumer goods companies from around the world announced an initiative recently that aims to simplify date labels worldwide. The group, which includes large companies such as Walmart, Kellogg, Campbell Soup and Amazon among others, is urging retailers and food producers to simplify date labels to reduce consumer confusion that has resulted in 1.3 billion tons of food being lost or wasted per year globally.
This effort corresponds with one announced earlier this year by the Grocery Manufacturers Association (GMA) and the Food Marketing Institute (FMI), which called for the use of two common phrases: ‘Best If Used By’ and ‘Use By.’
While the primary goal of this effort is to reduce food waste, attempts to simplify date labels have food safety implications as well. The term ‘Best if Used By’ is meant to inform consumers of the quality of a food product; the quality may not be optimal, but it conveys that it is still safe to consume. The ‘Use By’ date applies to highly perishable products, and is meant to inform the consumer that these products should be consumed by the date listed on the package and disposed of after the ‘use by’ date.
Outlook
Much like the effort to reduce labeling errors for allergens, this industry-supported effort to simplify date labels is expected to resonate and be applied widely on a voluntary basis. Food waste is becoming a higher profile issue, is receiving increased attention from policy makers, and could be addressed in the 2018 farm bill. Efforts by industry to address this issue voluntarily represent an opportunity to avoid or minimize any attempts to impose additional requirements through legislation or regulatory action.
Conclusion
The rulemaking process underway on labeling, along with the industry initiatives, reflects a strong desire by consumers to know more about the foods they purchase. This has been an ongoing trend that is likely to continue.