

Step into the lawyer’s kitchen with food attorney Jennifer Allen, Partner at Zwillinger Wulkan, as she breaks down current organic food labeling regulations and requirements.

Step into the lawyer’s kitchen with food attorney Jennifer Allen, Partner at Zwillinger Wulkan, as she breaks down current organic food labeling regulations and requirements.

The final rule will become effective 60 days after it is published in the Federal Register. The compliance date for all persons subject to the recordkeeping requirements is two years after the effective date.

The proposed rule, if finalized, will implement a 10 cent per pound increase in color certification fees.

Undeclared allergens remain a leading cause of recalls with three new announcements in the past week.
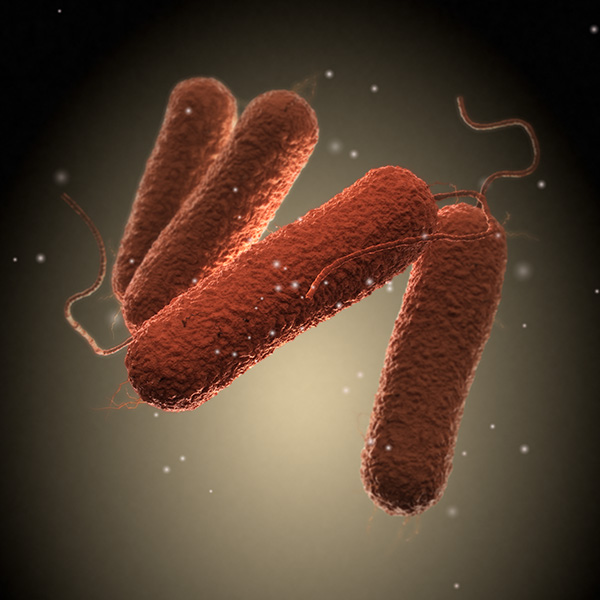
The National Advisory Committee on Microbiological Criteria for Foods is holding a public meeting on November 15 that will cover FDA action on Cronobacter spp. in powdered infant formula, updates under the Cyclospora cayetanensis subcommittee and the USDA proposed regulatory framework, “Enhancing Salmonella Control in Poultry Products.”

Through TAG India, the Acheson Group will provide expertise and resources to assist exporters in food safety efforts and domestic and foreign standards and regulatory compliance
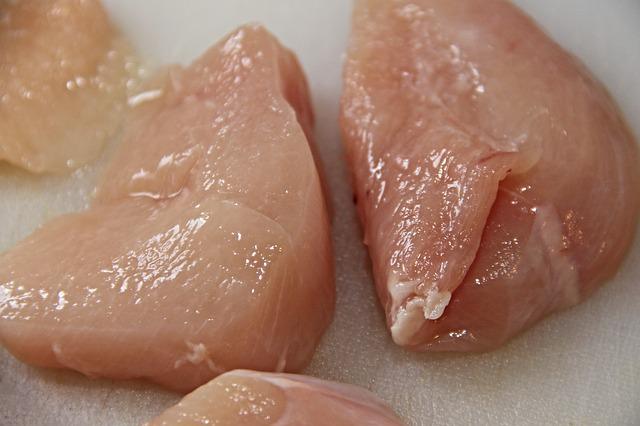
“We know that Salmonella in poultry is a complex problem with no single solution. However, we have identified a series of strategic actions FSIS could take that are likely to drive down Salmonella infections linked to poultry products consumption, and we are presenting those in this proposed framework.”

On October 21, the FDA will provide an overview of its proposed rule to update the definition of “healthy” nutrient content claims. On October 26, the FDA and Stop Foodborne Illness are co-hosting a virtual event on the use of rewards and recognition programs to drive positive food safety culture.

The FDA and CDC have signed a memorandum of understanding designed to help increase the consistency and capacity of retail food protection programs across the country, promote a general culture of food safety and facilitate continued communication between the FDA and CDC to assist state, tribal, local, territorial (SLTL) and industry partners.

In recent decades, the food manufacturing industry has seen a regulatory shift toward environmental monitoring testing. At the same time, it has been difficult for producers to build out environmental monitoring programs due to a lack of detailed regulatory guidance. Following are five strategies to help food producers develop more efficient and effective environmental monitoring programs that will help ensure safe finished products.