

Responsible sourcing is key to minding the food safety gap that allows contaminated protective gloves to enter the U.S. market.

Responsible sourcing is key to minding the food safety gap that allows contaminated protective gloves to enter the U.S. market.

Dr. Michelle Catlin has been appointed Chief Scientist of the USDA Food Safety and Inspection Services. She took on the new role on December 31, 2023.

Modern conveyor food processing systems include food-safe components and special features designed to maintain hygiene to prevent dangerous bacteria from contaminating products and limit their ability to spread. Following are key features to consider.

Cinnamon samples from Negasmart showed extremely high lead levels of 5110 ppm and 2270 ppm, as the FDA continues increased screening of imported cinnamon and its investigation to determine whether other products exported to the U.S. contain the cinnamon used in the recalled products.

The FDA has provided an update on actions the agency has taken, and those underway, to strengthen the safety and resiliency of the supply of infant formula, including a progress report on recommendations in the FDA’s Evaluation of the Infant Formula Response and strategies to prevent Cronobacter illnesses associated with infant formula.

Jennifer McEntire, Ph.D., founder of Food Safety Strategy and former Chief Food Safety & Regulatory Officer at the International Fresh Produce Association, shares what she has learned throughout her 25-year career in food safety.

The FDA has introduced new tools and answers to FAQs to inform stakeholders about the Food Traceability Rule and help covered entities come into compliance.
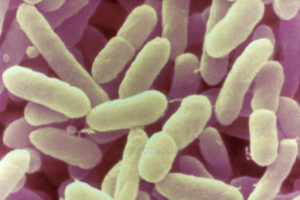
The recent large scale recalls of infant formula and resulting nationwide shortages highlighted the risks of Cronobacter in vulnerable populations. Sally Powell Price, Regulatory Expert Food and Beverage Safety at MilliporeSigma, which is the Life Sciences business of Merck KGaA, Darmstadt Germany in the U.S. and Canada, and Andrew Lienau, Food Regulatory and Validation Senior Expert at MilliporeSigma, discuss the risks of Cronobacter infection, a new testing assay that offers more rapid results, and coming changes to the regulation of infant formula due to growing concerns surrounding the dangers of Cronobacter.

This fall, Jim Jones stepped into his new role as the first Deputy Commissioner for Human Foods at the FDA. Here, he discusses his vision for the new unified Human Foods Program, what he learned from his work on the Reagan-Udall Expert Panel for Foods and why that led him to pursue his current role.

The ability to conduct a comprehensive root cause analysis to identify the origin of concerns and formulate and execute remedial processes, such as corrective and preventive actions (CAPA), is essential for food companies. Following we look at how to effectively conduct a root cause analysis and the benefits digital tools can offer teams in both identifying and preventing deficiencies.