

The CCT Tool, an online searchable database providing a consolidated list of contaminant levels (e.g., tolerances, action levels, and guidance levels) that are used to evaluate potential health risks of contaminants in human foods

The CCT Tool, an online searchable database providing a consolidated list of contaminant levels (e.g., tolerances, action levels, and guidance levels) that are used to evaluate potential health risks of contaminants in human foods

The USDA, FDA and HHS have announced efforts to combat the spread of H5N1 and provide financial support for lost milk production.

“Receiving approval from the CDFA for both meat and egg distributors in America and pig farmers in the UK allows us to provide international support to the animal products industry while increasing access to safe and traceable meat and eggs across the U.S.”

The guidance describes two processes through which companies may voluntarily inform the FDA of the steps they have taken to ensure the safety of foods from their genome-edited plant varieties: premarket consultations for higher risk foods and voluntary premarket meetings for lower-risk foods.

The Remote Grading Pilot for Beef allows a USDA grader to assess beef carcass characteristics and assign the official quality grade from a remote location, reducing costs and location as barriers to participation in voluntary grading services. The pilot program is part of USDA’s efforts to increase competition in agricultural markets and create a fairer playing field for small- and mid-size farmers and ranchers.

In 2022, CORE evaluated 65 incidents, responded to 28 and issued advisories for 11—a slight increase from 2021. The investigations in 2022 included E. coli, Cronobacter, hepatitis A virus, Listeria monocytogenes, and Salmonella which were linked to a variety of products, including produce, dairy and fish.

Dr. Michelle Catlin has been appointed Chief Scientist of the USDA Food Safety and Inspection Services. She took on the new role on December 31, 2023.

The FDA has introduced new tools and answers to FAQs to inform stakeholders about the Food Traceability Rule and help covered entities come into compliance.
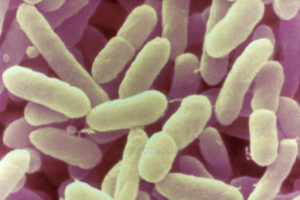
The recent large scale recalls of infant formula and resulting nationwide shortages highlighted the risks of Cronobacter in vulnerable populations. Sally Powell Price, Regulatory Expert Food and Beverage Safety at MilliporeSigma, which is the Life Sciences business of Merck KGaA, Darmstadt Germany in the U.S. and Canada, and Andrew Lienau, Food Regulatory and Validation Senior Expert at MilliporeSigma, discuss the risks of Cronobacter infection, a new testing assay that offers more rapid results, and coming changes to the regulation of infant formula due to growing concerns surrounding the dangers of Cronobacter.

The updates to the CFSAN Online Submission Module are intended to eliminate the need to use the FDA’s Electronic Submission Gateway (ESG) and to provide more secure communication between FDA and submitters.