Imagine this: While cleaning a slicing machine during a sanitation break, one of your employees discovered a piece of harp wire was missing from the machine. The meat that had been sliced since the cutting machine’s last inspection had already been added to your product, and the product had been packaged. It had already passed through your company’s inline inspection machines without any foreign contaminants being detected.
You enjoy a spotless reputation in the food industry and know that if consumers lose faith in the product, it will suffer significant damage to both its reputation and its bottom line. So what do you do now?
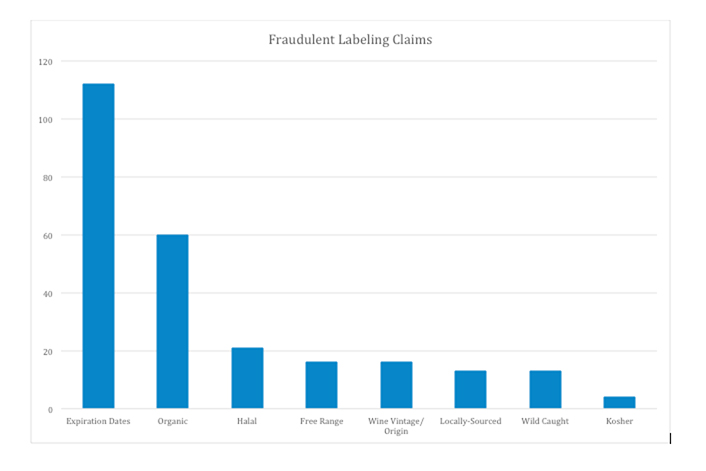
Here’s a look at four different scenarios and how each one can affect a respected food manufacturer.
Option 1: Dispose of the Full Production Run
Disposing of a full production run will give your company complete confidence that the contaminant issue is resolved and will never reach the public. However, you have to take into consideration the full cost and implications of such a move, such as:
Where to dispose of the contaminated product. The FDA has specific rules about disposing of contaminated food products, but those guidelines can be affected by local, state and even federal regulations. Among the disposal options are landfills, rendering or incineration. You must find the proper facility, arrange the safe transportation of the product and procure all the required permits for the disposal.
Food waste. With 40% of the food produced in the United States going to waste, and 50 million Americans not knowing where their next meal will come from, you don’t want to add to the problem.
Product out of stock. Having your product out of stock will be costly. In addition to the lost sales, there’s a chance that consumers will turn to another brand—and not return to buying your product.
Cost of reproduction. To re-run the entire product line, you will essentially double your costs. You will have to pay for the cost of the products used, as well as pay for new packaging and all labor costs.
Option 2: Rework the Product In-House
You can use your own resources and inline equipment to try and troubleshoot the problem. Running the product through the metal detector to look for the harp wire could salvage most of the product, and would give your company the ability to supervise the entire process. That way, if you find the metal, you’d know firsthand that the contaminated product is out of production and won’t reach consumers.
But there are some expensive downsides to this approach. Among the factors that your company must consider are:
Loss of productivity. Both from the standpoint of equipment utilization and the productivity of employees, reworking the product would be costly to the company. You would have to have to slow the production line and manually re-run all the product through the metal detector to look for the missing wire.
Increased labor costs. You would have to pay overtime to your employees and keep your standard production line running while it re-runs the product and looks for the suspected contamination.
Limitations of their inspection equipment. Your results are only as good as the equipment you are using, and there’s always a risk that the metal detector that missed it the first time won’t find it the second time, either.
It seems like a bit of a gamble; if the metal detector catches it the second time around, then it could be worth it. If the product is re-run and no contaminants are found, however, your company is back where it started and must decide how to move forward.
Option 3: Risk It and Ship the Product to Retailers
Since the metal wasn’t detected by the company’s inline inspection system, you cannot be absolutely sure the metal is in the product. You only know that a broken piece of harp wire is missing; it’s unclear whether that wire made it into the food.
The least expensive option—but also the riskiest—is to go ahead and ship the product, hoping that the missing wire didn’t make it into the food and, therefore, never gets discovered by consumers.
There’s a chance that the metal detector was right, the wire isn’t in the food, and things will be fine. However, if the risky gamble doesn’t go in your favor, the consequences could be severe and this becomes the most expensive option of all. Among the risks the company faces are the following.
Costly recall. Food recalls are always expensive. According to a study from the Food Marketing Institute and the Grocery Manufacturers Association, the average recall runs up a $10 million tab in direct costs alone.
This includes the cost of notifying the supply chain and consumers, retrieving the product, storage, disposal and additional labor costs associated with having to perform all of these actions.
Possible litigation. Food recalls often are accompanied by lawsuits, and if the metal wire is eaten by a customer and causes injury, you could be held liable for everything from medical bills to time lost from work due to pain and suffering.
Bad publicity and lost sales. In today’s 24/7 news world and with the power of social networking, news of a food recall can reach consumers at lightning speed. This equates directly to lost sales and can have a negative impact on your brand reputation and market value.
A recent Harris Interactive Poll found that 55% of consumers would switch brands temporarily after a recall, and 15% would never buy the product again. What’s more, 21% said they would avoid buying any other products made by that manufacturer.
Option 4: Use an X-ray Inspection Service
A fourth option can help avoid lawsuits, recalls and bad publicity, while at the same time sidestepping unnecessary waste and the costs associated with disposing of an entire production run or reworking it internally.
You can have your product shipped to an X-ray inspection facility, or use an X-ray inspection rental service.
A contaminant removal service and professional catalog reporting with full traceability could also ensure that the specific contaminant was located and removed, and you would have the confidence that the problem had been resolved as the product reached consumers. There are several other advantages to using a company that offers this type of solution as well, including:
Reduced waste. Because the only product being thrown away would be the product that was contaminated, there would be minimal waste. This is the only option that allows you to recover the rest of the product, with the certainty that it has been inspected and is safe for its customers.
Advanced detection capabilities. You can be confident in inspection process using custom technology that enables the detection of foreign particles down to 0.8 mm or smaller. In addition to metal, such systems can also detect product clumps, glass particles, stones, bone, rubber, plastic, wood, gasket materials, container defects and missing components.
This type of solution far exceeds the capabilities of inline inspection machines, and, because it can run a single pallet an hour, instead of the average 10,000 pounds an hour, and thus it spends more time focusing on what is passing through the machine to ensure no contaminants pass through.
Final Thoughts
When it comes to the quality of your product, it’s better not to take any chances. When you put your product line in the hands of a third-party X-ray food inspection company, you know you will get results since food safety is our specialty — and it’s what they do, all day, every day.