Some impressive technologies are not only impacting the food industry right now but will also have a huge impact in the future. As their use grows to be more prevalent, the industry will change to be smarter and more efficient, with continued improvements across the board.
1. AI and Advanced Robotics
While artificial intelligence and advanced robotics are two distinct technologies, they are frequently paired together. AI, and the data it digests, is used to command robots, allowing them to be more precise, more intelligent and more aware.
Most robots on their own are capable of completing only repetitive and clearly defined tasks. Throw something unique into the mix and they’ll either fumble or fail. However, when governed by data-based intelligence solutions like AI or machine learning, those robots become something incredibly advanced.
In the food industry, machinery and robots are leveraged to improve operations, further maintaining quality and efficiency, at affordable costs. They often work alongside human laborers to augment or enhance processes. They come with several unexpected benefits as well, such as much-improved safety for workers, faster and higher product output and consistent, reliable quality.
For example, JBS, one of the world’s largest meatpacking firms, deployed robotic butchers within its plants. The robots were used to slice more challenging meats, which reduced workplace injuries.
2. Automation
Automation stands alongside AI and advanced robotics, even incorporating those technologies to create a streamlined system. As of 2017, 73% of surveyed companies in the food and beverage manufacturing industry either had or were in the process of establishing automation within their facilities.
Many systems are designed to replace or enhance repetitive tasks, boosting their speed and accuracy, to significantly improve output, without incurring a loss in quality. It’s not just about hardware, like swapping a human laborer for a robot. It’s also achieved through software. Think supply chain management solutions that help plan for various events and experiences without human input.
When many of these technologies are used side-by-side, it strengthens their application and usability. As is true of advanced robotics, for example, AI can also be used to create more intelligent automation platforms. Instead of carrying out rote or simple tasks, they can be programmed to react and engage through any number of parameters. The system might slow production, for instance, based on a decrease in product demand. Or, it might swap to an alternate component or ingredient because of a shortage somewhere.
With the right controls and support, automation technologies are game-changing. With the global population growing and demands increasing more with each year, food manufacturers will look to streamline their operations and boost output in any way possible, and automation will be a go-to.
3. Digital Twins
Digital twins in food manufacturing are essentially simulated copies or a virtual representation of a physical system. That definition might seem confusing, but think of it as a clone that can be manipulated for testing and analytics.In other words, it is a twin of the actual system and information, in every sense of the word, albeit one that is more versatile and less vulnerable. It allows manufacturers and distributors to run simulations by feeding specific information into the system to identify patterns, recognize outcomes and much more.
As the systems and controls supporting the field become smarter and more digitized, digital twins in food manufacturing will find their way into product development, testing, post-production, distribution and nearly every other facet of the industry. It will become an integral component to not only understand what’s happening in the market but also for keeping up with the ebb and flow of supply and demand.
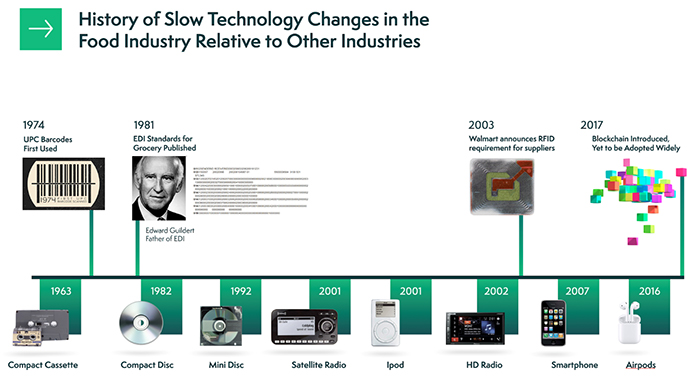
4. Blockchain
Even well before the pandemic, people had become much more conscious about the foods they consume. They want to know the origin of their goods and whether they’ve been sourced using safe, healthy and environmentally friendly methods. The problem with such demands is that, until recently, there haven’t been many solutions for increased visibility within the food supply chain.
Growing concerns for health are now a priority, and visibility is an absolute must. Blockchain technology is the answer, providing precisely the kind of visibility, efficiency, controls and collaboration that consumers want.
With this food manufacturing technology in place, someone could trace a head of lettuce back to its initial seeding. They can see who grew the plants and where, and which methods they used to mature the crop. Then, they can follow its journey to the store shelf.
How is such a thing possible? It all has to do with the technology. In its simplest form, Blockchain is a digital ledger or complete and digitized record of a particular data set. The data that goes in is added to something called a block, and as more is added, it is tacked on to the end of that block to create a long, linked record. Every bit of information is visible across the entire chain, hence the name blockchain.
Walmart is using the technology to track potential food contamination outbreaks. It empowers them to not just find the source but also find the many branches involved — like where goods might have been shipped and who may have purchased them.
Food Manufacturing Technology for the Future
While each food manufacturing technology discussed here is incredibly influential and will have a direct impact on the future of the industry, they are not the only solutions making waves. Some additional examples include:
- Drones and automated delivery vehicles
- 3-D printing for edible goods
- Smart or precision agriculture
- High-tech packaging
- Smarter waste disposal and recycling
The takeaway is that technology is vastly improving the operational efficiency of the food supply chain, from farmers and manufacturers to the retail stores featuring goods on their shelves. There’s no right or wrong buy-in, as any one of these technologies can be used to streamline separate processes. The biggest challenge will be deciding what to upgrade first, especially when it comes to delivering high-quality, fresh goods in a prompt manner.