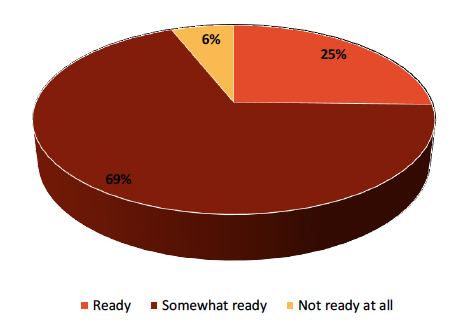
Many companies need to prepare for FSMA compliance by September, yet three out of four only consider themselves “somewhat ready”, according to a recent survey of about 400 food companies. However, the findings generally indicate that companies are taking action now to be FSMA ready—they just might need some help along the way.
Sponsored by SafetyChain Software and The Acheson Group, “2016 FSMA Readiness & Compliance Strategy” surveyed mainly U.S. companies (88% with more than $1 million in revenue) that produce and manufacture food for human consumption.
An effective food safety plan is a fundamental part of FSMA compliance. The majority of participants (80%) are either currently updating their food safety plans or plan on doing so this year. Conducting a gap analysis is a common way to assess the effectiveness of a food safety plan: 84% of respondents plan to conduct a gap analysis, have one in progress, or have already completed the exercise. More than half of these companies are tackling it using internal resources, and 29% are seeking expertise outside their organization.
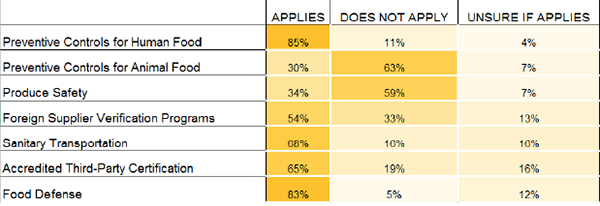
Documentation plays a large role in meeting FSMA requirements, and in the event of an audit, companies must be able to produce records within 24 hours. The good news is that 76% of companies say they can retrieve records required by FDA within this time span, but 22% are still unsure. Half of the organizations anticipate being audit ready on day one of FSMA enactment. And despite a push to migrate to electronic documentation, most of the companies surveyed (84%) still keep both paper and electronic records; only 3% keep strictly electronic documents.

The survey authors concluded that companies still need more information on how they can meet FSMA requirements, what resources are available, and how certain systems can help. In addition, they indicated that corporate executives must play a larger role in implementing compliance.