Ask any one in the food safety and quality sectors of the F&B industry and they all talk about one uniform concern: Audits. The sheer number of audits, supplier audits, GFSI audits, FDA audits, unannounced audits, the work involved in preparing for these, and the possibility of still things not working as planned on the actual day of the audit.
Dispelling some of these concerns, Gary Smith, who leads the strategic development and oversight of Eurofins’ Food Safety Systems division, including auditing, certification, and training programs, spoke to Food Safety Tech about how companies can be better prepared; challenges in moving from traditional GMP audits to GFSI recognized certification audits; role of management; and what do you if you disagree with the auditor.
Food Safety Tech: What is the biggest challenge for a company moving forward from traditional GMP audits to GFSI recognized food safety certification audits?
Smith: A good GMP audit program has probably got about 80 percent of the full GFSI scheme. It’s a great precursor. But there are some aspects that are not addressed in GMP audits which are facility-specific, such as would we know if the floor is in bad shape etc.? With GFSI you can’t have any non-conformance. Internal audit programs, corrective action management, root cause analysis, all of these are a must for GFSI. So it’s much more advanced than what’s required in GMP.
The audits are also difference from a validation stand point. For instance, with GMP audits, you need to have six elements of a Pest Control program and a facility can say, I have all six, so I am set. But with GFSI certification programs, you need to have these six elements, but you also have to prove that these are effective.
GMP audits provide a snapshot in time, a look behind the curtain to make sure you are following all the requirements. With certification, it’s a more consistent and continuous process – you are always looking for ways for improvement, and ensuring the standards and systems are working the way they are supposed to.
FST: What is the role of management in the audit process – whether GMP or GFSI?
Smith: There’s a big difference when it comes to management participation with both these sets of audits. Management needs to understand that with GFSI certification, management is required to have a culture of continuous improvement, where they are constantly looking for issues to manage and ways to get better. Leadership has to drive that change and a lot of folks struggle with this. QA managers should focus on training them to bring managers to embrace and communicate that culture.
FST: For companies first looking into certification services, what criteria should they be using while selecting a certification body?
Smith: The most basic requirement according to me is auditor availability. You need to identify a certification body that has more than one or two certified auditors. While price should be a consideration, it shouldn’t be the top priority.
As companies move to certification versus auditing, it is also important to look for a food safety partner, one who can service many of your foods safety needs such as testing, consulting, training etc., versus just auditing. For instance, can you call the Certified Body in case you have a recall or a food safety incident, and can the CB help you minimize the issue and solve the problem? Of course, customer service is an important consideration; the auditor and the team have to be responsive and polite.
FST: What steps can a company take prior to an audit to give themselves the best chance for success on the audit?
Smith: Training is critical. The facility and the personnel concerned with the audit process need to thoroughly understand the standard against which they are being audited, what will be asked and assessed for. Companies need to be harder on themselves than the auditor will be. Get others in maintenance and product involved in the facility and ask them questions that an auditor may ask. Train them to answer those questions.
Have strong internal auditing programs so you are prepared. You should know your issues and some auditor shouldn’t be telling you what the gaps are. All this is time and energy consuming, but it’s worth it.
Taking pictures both internal and external can also help, and can be a great training tool. When you have actual pictures from the facility and the processes, there can’t be too much room for debate.
FST: If the company disagrees with an auditor or the audit findings, what should they do?
Smith: The first thing I would say is don’t be afraid to ask the auditor questions. It is okay to say, ‘show me in the standard where it says this is a nonconformance.’ Remember to ask them before they leave. In most cases, asking such questions can help solve 75 percent of the issues.
Auditors can help describe why something is a nonconformance. If as a facility there’s still disagreement, you can go to the CB and provide a written description with as much information as you can, specific to the standard, about why you disagree with the audit. You can do an investigation and in many cases, probably 40 percent of the time, the auditor could have made an error, and gone beyond the standard. Remember that for the CB also, it’s important for them to get it right. So sites shouldn’t be worried about asking auditors questions, and CBs should respond to them – all in the process of continuous improvement.
Unfortunately, in some instances, it gets escalated beyond the CB to the standard owner. Though it’s a really drastic step, it has happened with SQF. As the final step, the site can still contact the Accreditation body, the American National Standards Institute.
FST: What are some other concerns regarding audits against the backdrop of FSMA proposed rules and GFSI?
Smith: As the industry is still waiting for final FSMA rules, one requirement that is causing some concern is auditors having to inform FDA when they see a nonconformance during a consultative audit. There is some concern that such a requirement would discourage people to try and get better. Industry is also waiting to see how FDA views certification audits. Can this be a process to ease imports, or, for domestic suppliers, be a risk-reduction tool? For instance, if there are two food facilities that make the same product, one that uses a GFSI scheme and one that doesn’t, can the one that uses be considered lower risk and not require as much resources to assess risk? Can inspections be done less frequently for such a facility? Will FDA accept such factors in the final requirements?
Ultimately, we need to remember that certification in the food industry in the U.S. is only about six years old. We all need to collectively continue to drive the process forward.


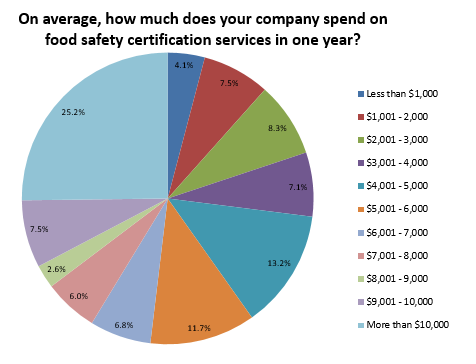
 Ultimately, the following factors influenced food companies’ decision to go through food safety certification:
Ultimately, the following factors influenced food companies’ decision to go through food safety certification: