I think many of us have once been – or are still – in a state of scurrying around right before an auditor knocks on our front doors. When you’re small and growing quickly, it’s hard not to be reactive for things like regulatory audits. But at some point, you have to proactively build out repeatable processes to drive long-term growth, and start really taking control of your own operation.
Here’s the ideal:
You want your co-pack operation to be in a state of audit-readiness. That is, if an auditor were to walk through your site today, you’d be proud of its set-up and its ability to uphold quality and regulatory standards for yourself and your client.
Earlier in October, I presented a webinar on “How to Run an Audit-Ready Co-Pack Operation,” i.e., how to get your house in order. Here are the five pillars of maintaining a state of audit-readiness for your co-pack operation:
Culture
In the same way that you have built a culture of collaboration and client satisfaction you’ll also need to build one for Quality. The way you prioritize and permeate Quality initiatives throughout the organization, from your senior team to your shop floor staff, will show when the auditor walks through the doors.
Paperwork
It’s vitally important that your paperwork is in order, including your SOPs, transactional items like RFQs, POs, and SOWs, and most importantly, the batch record. The auditor will be looking for two things amongst all of your documentation: content and consistency. The content needs to meet regulatory requirements, and there needs to be consistency between what you say you do and what you actually do.
It’s not rocket science: you need to write out what you do, and then you have to do the activities that reflect what you’ve written down. This is often the one thing that won’t be maintained properly, unless it’s given attention.
Physical Space
Your physical space will be audited to ensure it’s set up with the correct flow, that it is kept clean, and there is documentation to that effect.
To make sure your physical space is up to par for an audit, imagine that you yourself are the product… go back to your receiving doors and physically walk through your facility, as a “day in the life” of your product. You’ll be able to see where you go, how you are handled, and where each step is documented. Along the way, anything that’s not delineated, not treated or identified properly, or does not follow a logical flow, are the areas you need to lock down before an audit.
Material Control
An auditor will want to see that you’re in control of your materials, and that you have track and traceability. Being able to track where you’re storing things in the site, where it’s moving, what your processes are for moving inventory, etc… all that demonstrates that you have the traceability controls to be able to handle mock and real recalls and maintain consumer safety.
Production
Ahh… the production line. It’s where the magic happens. When setting up your production line, you need to make sure you have appropriate line clearance, the lines are segregated, the correct staff are on the line to do the project, and that they have had the proper training for their particular activity in the line. In the co-pack world, where things are highly customized with low repeatability, it can be easy to overlook the extensive amount of training it takes to make sure each production line is in good hands. You need, however, to make sure you do your due diligence to maintain production accuracy, quality assurance, and regulatory standards.
In the webinar, I discuss each of these areas in greater detail. If maintaining a state of audit-readiness is an endeavor you’d like to pursue, watch the webinar for more details or access the slide deck by clicking here.








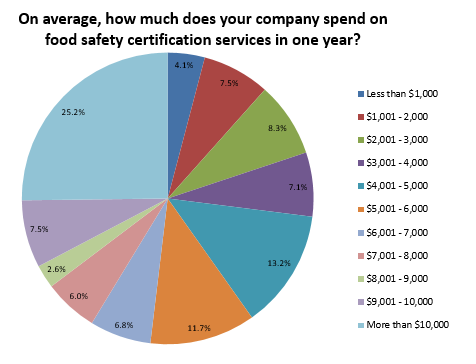


 Ultimately, the following factors influenced food companies’ decision to go through food safety certification:
Ultimately, the following factors influenced food companies’ decision to go through food safety certification:




 FST: You will also be talking about the direction of SQF in 2015 and beyond? Is there a “theme” or specific set of business drivers that are driving future changes to SQF?
FST: You will also be talking about the direction of SQF in 2015 and beyond? Is there a “theme” or specific set of business drivers that are driving future changes to SQF?