The food industry has been hard at work over the past few years implementing food fraud mitigation plans in response to Global Food Safety Initiative (GFSI) certification program requirements. GFSI defines food fraud as:
“A collective term encompassing the deliberate and intentional substitution, addition, tampering or misrepresentation of food, food ingredients or food packaging, labelling, product information or false or misleading statements made about a product for economic gain that could impact consumer health.” (GFSI Benchmarking Requirements, 2017)
GFSI then further defines the terminology of food fraud by citing seven categories (shown in the following diagram).
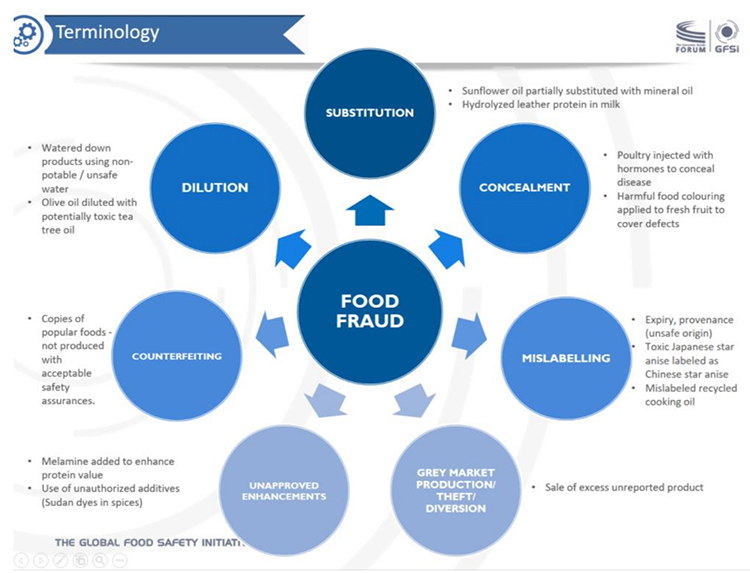
In the Food Fraud Database, we categorize food fraud records using the following terminology (with examples):
- Dilution/substitution
- Substitution of an entire fish fillet or partial dilution of olive oil with another oil
- Artificial enhancement
- Addition of melamine to artificially increase the apparent protein content of milk or the addition of coloring agents to spices
- Use of undeclared, unapproved, or banned biocides
- The use of chloramphenicol in honeybee populations (where not permitted) or the addition of hydrogen peroxide to milk
- Removal of authentic constituents
- The sale of “spent” spice powder (used in the production of an oleoresin) as a whole spice powder
- Misrepresentation of nutritional value
- Infant formula that does not contain the required nutritional content
- Fraudulent labeling claims
- Misrepresentation of label attributes related to production method (organic, kosher, halal, etc.)
- Formulation of an entirely fraudulent product (using multiple adulterants and methods)
- The sale of “100% apple juice” that consists of sugar, water, malic acid, flavor, and color
- Other
- This includes counterfeits, theft, overruns, etc.
Harmonization of food fraud terminology is frequently discussed, so I thought it might be useful to provide information on how our definitions relate to the GFSI terminology:
GFSI category “Dilution”: This category maps directly to our category dilution/substitution. The reason we combine these into one category is that the intent is the same: To replace the weight or volume of a product. This can occur either through partial or full substitution of a liquid product, a granulated product, or swapping an entire intact product such as a fish filet. One of the GFSI examples for substitution is “sunflower oil partially substituted with mineral oil”, which could just as accurately be described as dilution.
GFSI category “Substitution”: As noted above, this category maps directly to our category dilution/substitution. However, we would not consider the use of hydrolyzed leather protein in milk (one of the cited examples) to be dilution/substitution because it is not used to replace weight or volume. We would view that as artificial enhancement of the protein content of milk.
GFSI category “Concealment”: We do not include a category focused on concealment because all food fraud involves concealing some aspect of the true contents of the food. One of the examples cited in this category is “poultry injected with hormones to conceal disease.” The use of antibiotics, anti-fungal agents or other substances to reduce bacterial load or mask deterioration would be classified, in our system, as the use of undeclared, unapproved or banned biocides. The use of coloring agents on fruit to improve appearance would also be classified as artificial enhancement.
GFSI category “Mislabeling”: Since all food fraud is, to some extent, mislabeling, we reserve the use of the term fraudulent labeling claims to those label attributes that describe production processes (organic, kosher, etc.). With the exception of falsification of expiration dates, the other examples cited would not be classified by us as mislabeling. The sale of Japanese star anise, which is potentially toxic, as Chinese star anise (a different species) is dilution/substitution and a health risk to consumers. The sale of cooking oil that has been recovered from waste streams and illegally produced is also a form of substitution that poses a potential health risk to consumers.
GFSI category “Unapproved enhancements”: This GFSI category aligns nicely with our category artificial enhancement, and both examples cited are nicely illustrative of the concept, which involves the fraudulent addition of a substance specifically for its function (not as a replacement for weight or volume).
GFSI Category “Gray market production/theft/diversion”: The production and sale of food products through unregulated channels would all be classified in our category called other. Because these forms of food fraud involve the sale of food outside of regulatory control, prevention measures will generally be substantially different from the prevention of fraud within legitimate supply chains.
GFSI Category Counterfeiting: This GFSI category is similar to the gray market production/theft/diversion category in that it involves intellectual property infringement and production outside of regulatory control. It would similarly be classified in our other category.



