Many food suppliers are investigating and making early investments in the adoption of digital technology to aid and automate their food safety programs. One area of intense interest has been the increasing application of digital automation within food safety testing programs. As a data and technology leader and practitioner across multiple industries for the past 30 years, I have had the privilege of working with organizations as they seek to build the appropriate plans and business justification for taking on digital transformation initiatives.
The following are the top three questions I am continually asked and the answers that, over time, have provided the information and support needed to help companies through this important transition. Perhaps these are similar to the questions that your leadership is asking you?

What are the actual benefits that digitizing our food safety testing program will yield?
David: During the past six years, I have witnessed the implementation and deployment of approximately 500 digital food safety testing programs across the globe. This is always one of the first questions I am asked, and there is inevitably a challenge in the question… namely – “Our program is solid, we pass our certification and customer audits, so tell me how this will be better than what we are doing today.”
The most tangible benefit, of which there are many, boils down to two metrics of success that I have witnessed consistently, over time and across three decades of work, deliver real, measurable change: Time and Trending.
Time, metrics within food safety scenarios, is characterized as having three components:
- “Time-to-information” – reducing the time between an event occurring, and information about that event being communicated to those in need of this knowledge.
- “Time-to-decision” – reducing the time from when information is known to the moment when a decision can be made to affect the outcome of whatever the information indicates as needing to be addressed.
- “Time-to-Action” – reducing the time from when a decision is made to when the enactment of that decision is carried out.
In the world of food safety, the diagnostic information, policy- and compliance-driven decisions, and the corrective and preventative actions (CAPA) that are mandated by regulation and driven by policy compliance standards, define the effectiveness of a food safety testing program.
Therefore, the ability to decrease these timeframes immediately produces a risk reduction result. When the time between an occurrence of a food safety issue and the completion of a corrective action is reduced, so too is risk reduced. Especially when the issue is deemed to be impacting public safety, brand value, and operational continuity… all of which can yield very costly results if not addressed quickly and accurately.
If information is collected and recorded manually, and is stored in individual paper and spreadsheet files, it is, by definition, made ineffective. The time it takes to manually record the data, or retrieve it when needed, works against the requirement that information be on-tap in real time as issues arise. Digitizing a food safety testing program means, beyond merely putting data into spreadsheets, that information be collected and stored in a data base, whether that is data coming from on-premises testing, or a 3rd-party lab. And that database must be connected to a system that enables immediate access, auto-alerts, and data-driven triggers to enact corrective actions without having to wait for a human to think where to look, or how to combine data from various manual storage files.
Trending is equally important. While speed, as highlighted above, is critical, so too is the continuous collection and analysis of data. The ability to trend your diagnostic results goes further than merely seeing a time series report of testing results. If constructed properly, a trending analysis can provide your team with the ability to become far more preventative than ever before.
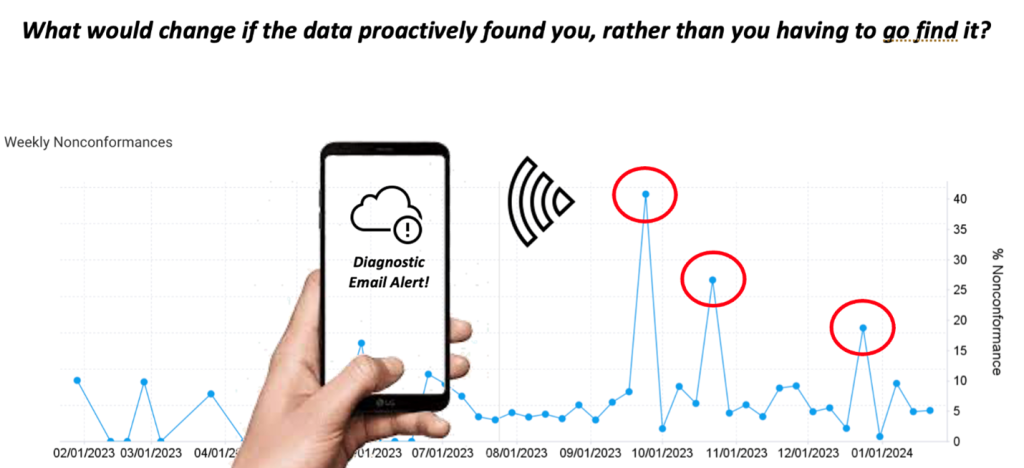
Trends allow you to see reoccurrences of issues, and as these grow, an alert engine can offer recommended actions that can prevent the future need for full CAPA scenarios, or worse, response to a regulatory inquiry. As illustrated below, when combined together, Time and Trending can yield significant benefit that, as a result, reduce a significant portion of the risk and costs associated with slow response to manually managed food safety testing data. Further, a digitized system can put your data to work for you, creating a scenario that enables the data itself to find the right person at the right time when thresholds and triggers deem this action to be necessary (see figure below).
How can we measure Return-on-Investment (ROI) on the cost of moving to a digitally managed program?
David: If the question and answer above are not yet enough to justify the move to a digitally managed testing program, there are three more factors to consider when assessing the return on a digital investment:
Reduction of production downtime: The occurrence and frequency of food safety issues increases the risk of system downtime. If a pathogen detection occurs, a machine, conveyor system or other equipment may need to be temporarily shut down for unplanned cleaning, or in extreme cases, torn down altogether for deep cleaning. Our interactions with over 500 implementations have shown that:
- Downtime can reach an astounding 500 hours annually, leading to overall costs that some studies put in the range of $20,000 to $30,000 per hour, on average.
- The financial impact of reducing production downtime by just 90 minutes per week can be dramatic once you’ve added up the week-after-week results. For example, a company operating two facilities with a $30,000/hour downtime cost can gain back $90,000 per week with just 90 minutes regained at each location weekly.
Reduction of Waste/Scrap and Rework: A pathogen positive diagnostic result in a Zone 1 or food contact surface location, or worse yet, within a finished product test, will result in the need to scrap and rework production lots. Each pound of finished product that is scrapped will require rework to make up for lost order fulfillment for customers. It is therefore imperative that when issues are detected, the associated corrective actions quickly and accurately address the situation. In my experience, we show that digital trending and time-to-action improvements can drive business impact – specifically, gaining back just 10% of scrapped food per week can yield significant results. For example:
- An organization operates two facilities where 500 lbs. of finished product are scrapped each week.
- The value per pound of finished product, when factoring in all the labor, energy costs and materials, is valued at a conservative cost of $1 per pound.
- Annually, by reducing time-to-information by just 4 hours per sanitation cycle, the organization was able to realize $400,000 reduction in waste-related costs.
Improve Overall Efficiency: Over the course of several months, we partnered with a large dairy producer to explore how automating a manual EMP process could help drive increased efficiencies, reduce pathogen positives, and ultimately, improve the bottom line (see figure below).
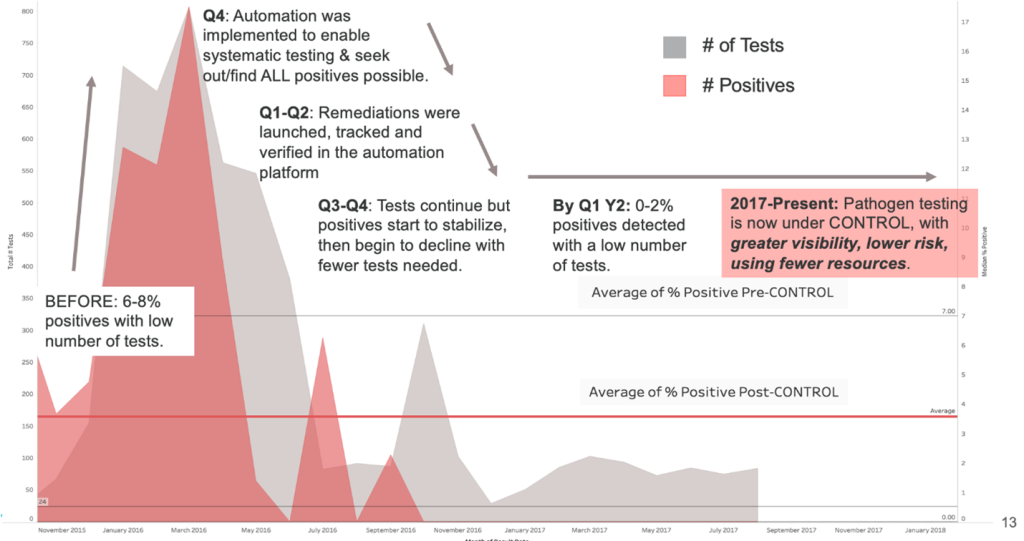
Over time, the analysis gained from automated data gathering enabled new sanitation procedures to implemented, leading to significant efficiency gains:
- A new baseline of testing volume, test types and correlated sanitation procedures were refined and implemented.
- A revamped remediation program yielded new corrective action steps that have been proven through the study’s data to be more effective.
- The FSQA team gained back 25% of their time by eliminating the need for manual reporting, analysis, and spreadsheet-based data preparation.
- The organization improved corrective action completion time by 50%.
What are the resources and time required for the transition to a digitally automated program?
David: There can be a high degree of ‘fear of change’ involved in any digital transformation initiative. This is not unfounded fear, as horror stories abound regarding large enterprise system implementations and the havoc they can cause. The main consideration in avoiding these outcomes is to ensure the initiative has leadership buy-in and support. This is why the answers to the first two questions above are so important. The path to gaining leadership buy-in is through the ability to connect food safety digitization and automation to tangible business results. If a successful business case can be made utilizing the concepts described above, then the battle for assigning resources and the appropriate implementation timeframes can be achieved.
I started working with food safety teams in 2018, when the existence of food safety testing automation was still at a relatively low adoption rate. In the intervening six years, as adoption has increased, the complexity and timeframes of implementation have decreased significantly. This remains a key area of concern, however, as organizations are struggling to keep up with ongoing staffing shortages and resulting resource constraints. There are two key areas where a digital solution provider must be challenged to prove their ability to support your digital transformation:
- Proof of delivery: Due to the relatively recent emergence of digital food safety testing platforms, we have not yet reached a state of maturity where tens of thousands of implementations have defined a standard of known implementation and adoption processes to exist. Therefore, it is critical that you find and work with a provider who will deliver a fully functioning trial of their system, preferably free of charge, for a significant amount of time. This will enable you and your team to experience the full range of capabilities offered, including the onboarding and training program, the length of time it takes, the level of technical acumen your team will be expected to have, and the overall delivery of the benefits described above.
- Focus on requirements: Commonly, digital solutions are designed to work within fairly rigid processes and workflows as designed into the system. If you’re lucky, you may find a solution that aligns to your own existing workflows, but all too often, the largest stumbling block is the realization that your new system is not just a digital transformation but also requires a full business process reengineering project in order to conform to the way the software works. Challenge your providers to demonstrate how their solution is flexible enough to enable your team to reduce any process changes to the lowest degree possible. While some new processes will always be inevitable (and potentially helpful!), it should be the hallmark of any provider’s customer support/success team to understand your requirements and configure their solution to enable them without too much drastic change.
To learn more about how digitizing food safety programs can impact business ROI, download our EMP ROI white paper today.
Visit Neogen to learn about digitized food safety