In the U.S., allergens are the sixth leading cause of chronic illness with over 50 million people affected annually.[1] This is just one country among many that has seen the steady increase of the prevalence and severity of food allergies.[2]With increased media attention on this growing issue, food product labeling and allergen testing have never been under such scrutiny.
In food product labeling the stakes are high. Consumers need to know that the ingredient lists of the products they buy are accurate, and manufacturers need assurance that their foods are allergen-free, especially as current labeling guidelines lack clarity.
Food testing methods, which have hitherto been deficient in detecting and identifying unknown allergens and cross-contamination within the food manufacturing process, must be further developed to become quicker and more precise, cost effective, and user-friendly. New analytical tests can identify a broader range of potential allergens and offer food manufacturers a way to detect emerging allergens.
The Pressure for Improved Regulation
With the number of people suffering from food allergies in the U.S. doubling in each of the last two decades,[3] there is a high demand for food manufacturers to improve the allergen information they provide for consumers.
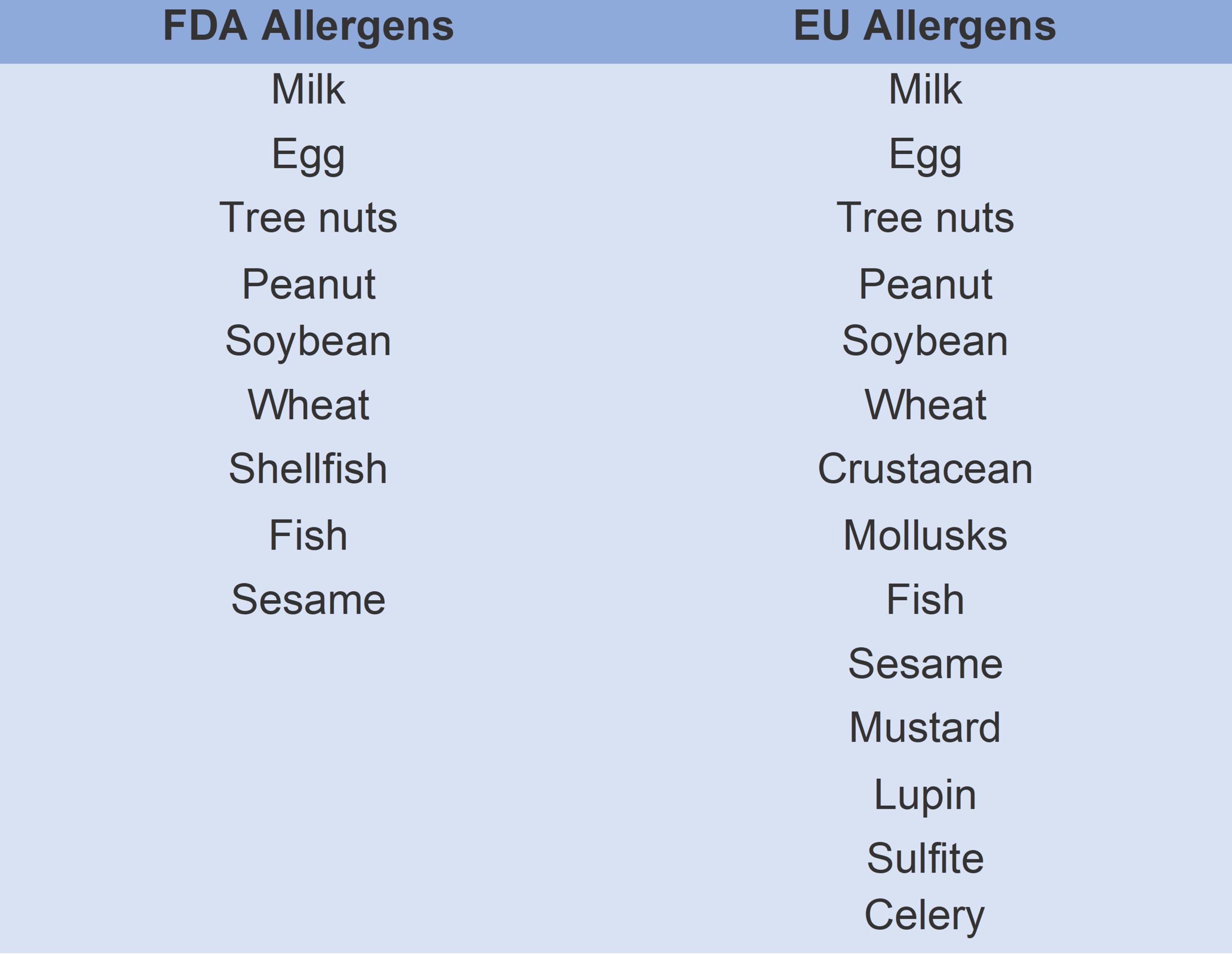
However, there is a lack of uniformity across different regions. While European Union law stipulates that allergens must be listed in bold on product ingredient lists, only 14 of the 200 foods that could potentially cause allergic reactions are prioritized[4] and, in the U.S., the FDA lists only nine key allergens.[5] Furthermore, while organizations such as Anaphylaxis UK agree that the most severe allergic reactions are caused by the consumption of a certain quantity of an allergen, there is no universal agreement on what such threshold levels should be.[6]
Single ingredients are not the only cause of allergic reactions: contamination and cross-contamination can occur at various stages of the food manufacturing process and put consumers at even greater risk.[7] Many manufacturers voluntarily use Precautionary Allergen Labeling (PAL) on their products to mitigate the risk of undeclared allergens, which may be used when there is a risk of allergen cross-contamination in the supply chain.[8] An example of PAL is ‘may contain milk’. This is not a legal requirement, however, and PAL protects the manufacturer more than the consumer as it is unlikely to be based on an assessment of the risk of cross-contamination for each of the 14 regulated allergens.[9]
Current Allergen Testing Options
Enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR) are the two main analytical methods presently used to detect and quantitate allergens in routine food testing.[10]
Highly sensitive, the ELISA method allows for the quantitative and qualitative analysis of antigens, proteins, hormones, and antibodies in biological samples. It is most commonly used in the identification of foodborne microorganisms, such as Salmonella and L. monocytogenes, across a range of food products.[11]
Although ELISA can identify specific analytical targets, it cannot detect unknown allergens in contaminated food supplies. Moreover, the overall structure of proteins and their extractability is often altered during food processing, which can affect assay test results. Other factors, including poor comparability of results between test kits that use different antibodies, can also impact each test, and therefore make reproducibility between methods difficult.
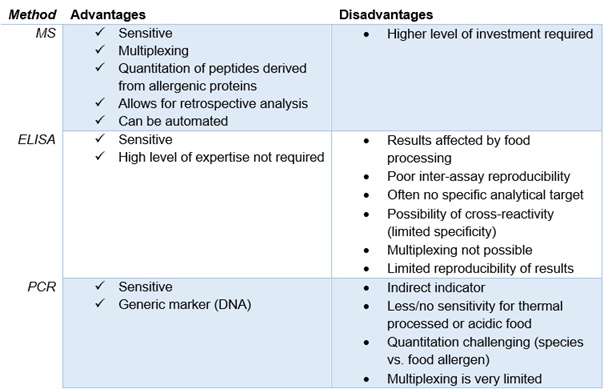
PCR testing, which uses deoxyribonucleic acid (DNA) as a genetic marker, is popular because of its sensitivity and specificity, and also because sample preparation is standardized. A limitation of PCR testing is that, as an indirect indicator, it lacks sensitivity for foods that could contain high quantities of allergenic protein, but little to no DNA.[12]
Liquid chromatography-mass spectrometry (LC-MS) is not commonly used in current food testing. Unlike ELISA, LC-MS has multiplexing capabilities, allowing for the detection of multiple allergens in a single run. It can provide precise separation, identification, and quantification of the specific peptides rather than proteins in samples, which not only increases test accuracy, but also improves upon traditional testing methods by allowing for the differentiation of closely related allergens.
However, LC-MS, too, has limitations. The complex matrices of some biological samples can cause issues, and the various sample techniques needed for specific analytes mean that implementing a LC-MS workflow in routine testing laboratories is difficult. Professor Jens Brockmeyer and his team at the University of Stuttgart have been working on further advancing LC-MS to mitigate such drawbacks.
The Solution for Comprehensive Allergen Testing
A research team at the University of Stuttgart is investigating the influence of food processing on allergenic potential. The team is seeking to improve the methods used to screen for allergens primarily through the use of mass spectrometry (MS) and has developed a new workflow for allergen testing that delivers results quickly and efficiently.
There are three components to the novel method: sample preparation, analysis by high-performance liquid chromatography (HPLC) coupled to MS, and data analysis software. The approach can be used in the analysis of food products and allergens, making sample preparation easier and simplifying laboratory workflows.
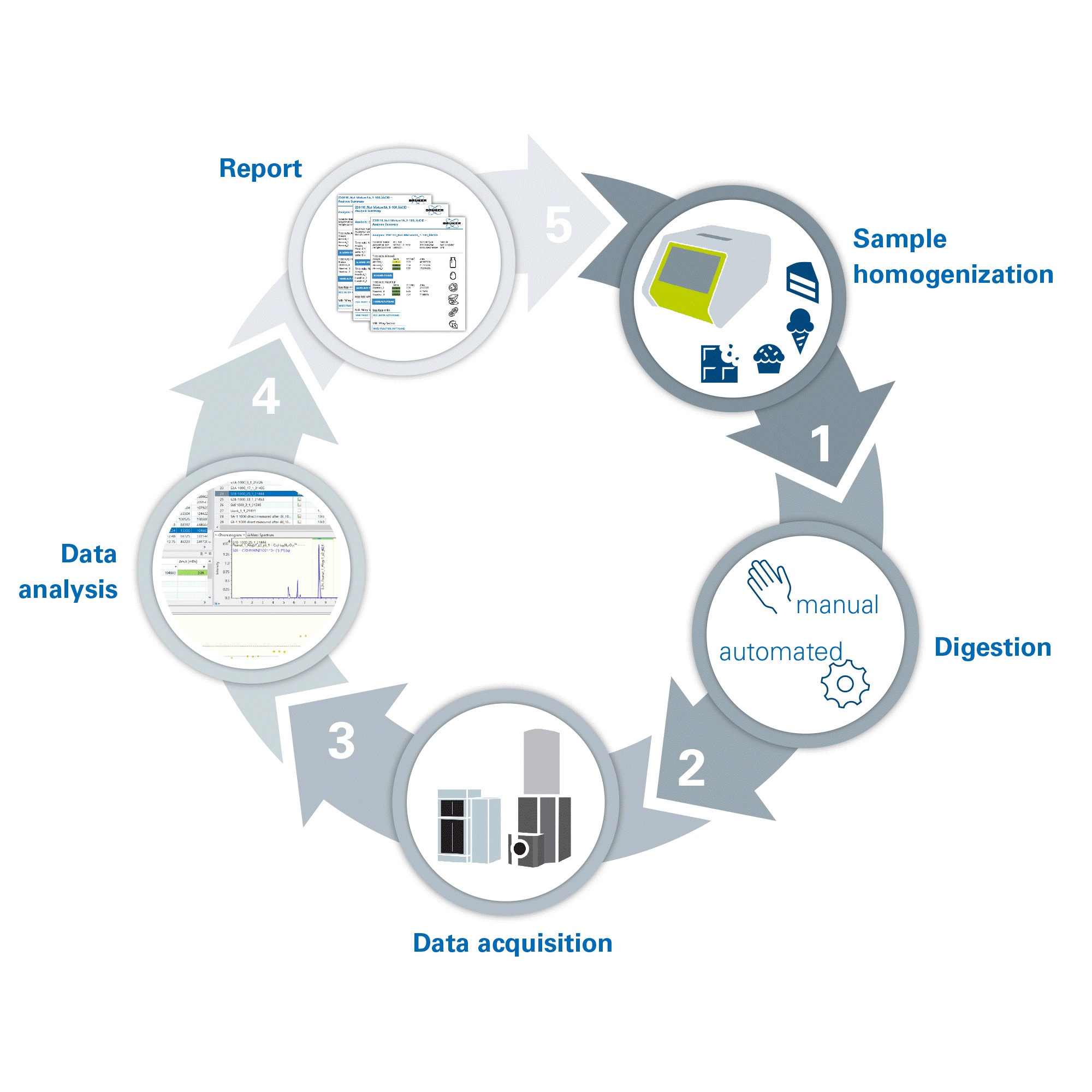
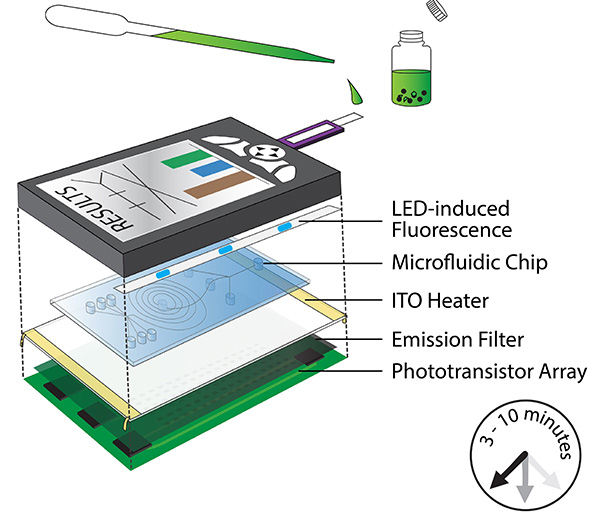
Protein extraction, manual or automated enzymatic digestion, and the cleanup of peptides takes place to prepare the sample. Protein digestion takes three hours, a considerable time saving in comparison to the usual proteomic procedures.[13] HPLC-MS is used to generate data; the detection of precursor masses of the specific peptides resulting from the allergenic proteins of the given ingredient verifies the presence of each allergenic element.
The new multiplexed method removes the need to run multiple tests to identify each allergen individually; just one run is required for the detection of several allergens. This multiplexing capability and the analytical software’s ability to evaluate measurements retrospectively significantly accelerates experiment time.
Improved Visibility of Unknown Allergens
Current food testing methods, such as ELISA and PCR, are valued for their sensitivity but both lack multiplexing capabilities and produce results that are affected by food processing and thermal processing, respectively. HPLC-MS is an innovative method that offers multiplexed analysis of complex samples, producing standardized results in an accelerated timeframe suited to the high throughput needs of food testing laboratories.
With undisclosed allergens the cause of 42% of food product recalls in the U.S. in 2022,[14] the precision and speed of HPLC-MS offers exciting potential for the future of food allergen testing, paving the way for the feasible implementation of clearer and more stringent regulations.
References
[1] American College of Allergy, Asthma, & Immunology. Allergy Facts. Accessed at: https://acaai.org/allergies/allergies-101/facts-stats/. Last accessed: November 17, 2023.
[2] The Guardian. ‘It’s one of the great mysteries of our time’: why extreme food allergies are on the rise – and what we can do about them. Accessed at: https://www.theguardian.com/society/2023/jul/15/its-one-of-the-great-mysteries-of-our-time-why-extreme-food-allergies-are-on-the-rise-and-what-we-can-do-about-them. Last accessed: November 17, 2023.
[3] Food Allergy & Anaphylaxis Connection Team. Food allergy & anaphylaxis. Accessed at: https://www.foodallergyawareness.org/food-allergy-and-anaphylaxis/prevention/food-allergies-on-the-rise/#:~:text=The%20number%20of%20people%20with,identified%20food%20allergy%20(source). Last accessed: December 21, 2023.
[4] European Union. Annex 2 – allergen labelling. Accessed at: https://food.ec.europa.eu/system/files/2018-07/codex_ccfl_cl-2018-24_ann-02.pdf. Last accessed: November 15, 2023.
[5] U.S. Food and Drug Administration. Allergic to sesame? food labels now must list sesame as an allergen. 2023. Accessed at: https://www.fda.gov/consumers/consumer-updates/allergic-sesame-food-labels-now-must-list-sesame-allergen. Last accessed: November 8, 2023.
[6] Anaphylaxis UK. Allergen thresholds. Accessed at: https://www.anaphylaxis.org.uk/fact-sheet/allergen-thresholds/. Last accessed: November 16, 2023.
[7] Food Allergy Canada. Avoiding cross-contamination. Accessed at: https://www.foodallergycanada.ca/living-with-allergies/day-to-day-management/avoiding-cross-contamination/. Last accessed: November 16, 2023.
[8] Food StandardsAgency. Precautionary allergen labelling. Accessed at: https://www.food.gov.uk/business-guidance/precautionary-allergen-labelling. Last accessed: November 16, 2023.
[9] Food Standards Agency. Precautionary allergen labelling. Accessed at: https://www.food.gov.uk/business-guidance/precautionary-allergen-labelling. Last accessed: November 16, 2023.
[10] Allergen Bureau. Food allergen analysis. Accessed at: https://allergenbureau.net/food-allergens/food-allergen-analysis/. Last accessed: November 16, 2023.
[11] Law JW-F, Ab Mutalib N-S, Chan K-G, Lee L-H. Rapid methods for the detection of foodborne bacterial pathogens: Principles, applications, advantages and limitations. Frontiers in Microbiology. 2015; 5.
[12] Stoyke M, Becker R, Brockmeyer J, et al. German government official methods board points the way forward: Launch of a new working group for mass spectrometry for protein analysis to detect food fraud and food allergens. Journal of AOAC International. 2019;102(5):1280-1285.
[13] Switzar L, Giera M, Niessen WM. Protein digestion: An overview of the available techniques and recent developments. Journal of Proteome Research. 2013;12(3):1067-1077.
[14] U.S. PIRG Education Fund. Food for thought part 2: An analysis of food recalls for 2022. Accessed at: https://pirg.org/edfund/resources/food-for-thought-an-analysis-of-food-recalls-for-2022/. Last Accessed: December 21, 2023.