Barbara Levin, Senior Vice President and Co-Founder of SafetyChain Software, shared her thoughts during a recent interview with Food Safety Tech.
Food Safety Tech (FST): Why are food safety audits such a hot topic of conversation?
Barbara Levin: Audits are a critical component of any food safety plan, and I’ve never heard food safety and quality assurance (FSQA) folks disagree with that assumption. But between regulatory, 3rd party standards such as GFSI, customer and internal audits – most of which are still very manual processes – audit preparation and response have become a huge manual burden that can be highly disruptive to operations. And while all audits has some commonalities, each has its own specific requirements as well, adding to the challenge.
In conversations with our clients at SafetyChain, we have heard of companies that have as many as 300 audits a year! So it’s a struggle to manage these audits while also having to get product out on time, within operational Key Performance Indicators, and of course meeting safety and quality requirements. This is why we’re also hearing more about technology solutions that can help companies be audit ready. But whether or not a particular solution is right for your company depends on how you define audit readiness.
FST: Before we discuss the definition of audit readiness, you mentioned that each type of audit has its own requirements – can you highlight some of these?
Levin: Let’s begin with what all of the audit types have in common – which fall in to four areas:
- First, you have to say what you do – all of your SOPs, PRPs, GMPs, HACCP/HARPC components, etc.
- Second, you have to verify that you do what you say.
- Third, you have to validate that it works.
- And last, you have to ensure that everything is documented.
On top of these commonalities, each audit type has some specific requirements. For example:
With regulatory audits, USDA can ask for pre-shipment reviews, while FDA can do unannounced audits with just a two hour notice.
- With the GFSI schemes, you have to have an approved vendor program and be able to demonstrate management commitment and continuous improvement. And of course everyone is talking about the upcoming SQF unannounced audits, which, I personally think, is something that the industry should embrace.
- With customer audits, it’s not just about safety, but also quality attributes such as weight, moisture or salt content to name just a few.
- And internal audits can be the hardest of all as many of the above elements, among others, can get combined.
FST: Given this wide range of requirements, what then, do you mean, when you say “true audit readiness?”
Levin: When you hear people talk about audit readiness, and audit automation solutions, the conversation is often focused on documentation – the ability to produce electronic records. And this is of course an important component of being audit ready. But in my view, true audit readiness goes far beyond documentation. It should also mean that you have the tools and processes in place to ensure that you actually PASS your audits with flying colors! It means that food safety systems have been consistently and diligently followed across all facilities; you have a robust supplier compliance program; non-conformances are caught at the earliest point possible, and CAPAs have been put in place; you have easy access to data for continuous improvement; and everything is documented. In other words, it’s not enough to just show that the paper has been gathered for the audit – but that you are doing the right things for food safety every single day. And if we’re talking about audit automation technology – these solutions should support all of these components.
FST: How can companies assess if they’re truly audit ready?
Levin: Here are some basic questions FSQA teams should ask themselves:
- Are we 100 percent sure that all SOPs, CCPs, PRPs, GMPs, etc., are current and being carried out, and that we can easily access verifying documentation?
- Do we have a robust supplier compliance program to ensure vendors are meeting our safety and quality requirements? Can we easily access all of those records?
- Are we getting non-conformance alerts in a timely manner to take corrective/preventive actions before product goes in to commerce? Can we easily access proof of CAPAs?
- Do we have easy access to all of the data required for trending, hazard analysis and continuous improvement?
There can be three answers to these questions: Yes, No and Hmmmmm. If you’ve had one or more No’s or Hmmms… chances are you may not be as audit ready as possible.
FST: What are then the challenges to being truly audit-ready?
Levin: I would list the biggest challenges with being audit ready as falling into four key areas:
- The volume of forms, records and paper that needs to be current, managed, easily accessed and actionable – meaning the data from these records can be trended for continuous improvement;
- Ensuring that all food safety programs are being carried out correctly and consistently – including the ability to catch problems at the earliest point possible, put a corrective/preventive action in place and make sure that all of that is documented;
- Management of supplier compliance (are you sure your suppliers are following all of your requirements?) and approved vendor program management; and
- The amount of time it takes to prepare for audits – especially as unannounced audits become more prevalent.
FST: How can automation help with these challenges?
Levin: Technology solution that helps companies be audit ready must go beyond document management. They have to integrate supplier/vendor management; food safety and quality program management – HACCP and HARPC programs, for example; process management and workflow; GFSI program compliance; and, of course, ensure that all records and documentation is available in a central repository for trending, continuous improvement and of course audit readiness.
These solutions should automate, streamline and improve FSQA. And the final result has to be that actionable data – across all products and facilities – that allows you to find ways to improve processes, put preventive controls in place and make continuous improvement an inherent part of company’s overall food safety culture. Audit readiness then becomes the benefit – not just the goal.
If you are using a cloud based FSQA automation solution – with roles-based security – automation can provide the kind of transparency and visibility that can actually reduce the amount of audits a company has by allowing suppliers, auditors and customers to view various slices of data. For example auditors might see non-conformances and the documented CAPA; a customer could see a finished product review; and a supplier can see that their Certificate of Analysis was received and met specifications. That’s a true change in traditional culture, but we’re seeing it more and more.
Food companies need to remember that auditors do not expect to see that everything thing was perfect all the time. But what they do want to see is that a problem was found in a timely manner and that it was fixed before the product went into commerce. Bottom line? Automation can help food and beverage companies say what they do, do what they say, make sure it works and make sure it’s documented and actionable. And when all of this information is easily organized by the type of audit – and cloud-based – companies can be audit-ready on-demand!
Click here to read Barbara Levin’s paper on how to be audit ready, on-demand with Food Safety Chain Management Systems.


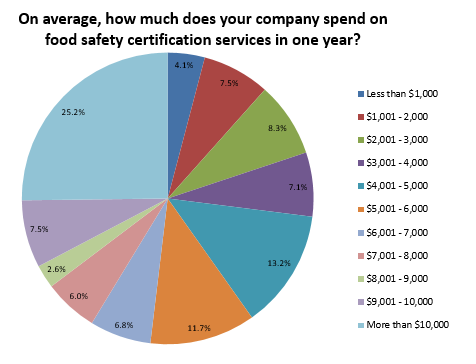
 Ultimately, the following factors influenced food companies’ decision to go through food safety certification:
Ultimately, the following factors influenced food companies’ decision to go through food safety certification: