The food industry has multiple levels of people involved, ranging from leadership and supervisors, all the way to line and front-end workers. Training, thus, has to not just give directions, but also provide better understanding of why we do what we are supposed to, says Gary Smith at Eurofins.
Smith, who leads the strategic development and oversight of Eurofins’ Food Safety Systems division, including auditing, certification, and training programs, says that “training for supervisors and leadership usually works well. But training workers is fraught with challenges. General training is focused more on giving directions, but not so much on providing understanding. People often skirt this rationale with the line workers, or it’s lost in translation, or missed out due to time constraints, turnover, vacation days etc. That’s where training fails.”
What would happen if we don’t spend this time address the ‘why’ behind training? People then get busy, and then miss out on steps and processes, and that causes problems, Smith replies. “It’s also critical to aim to strike a balance, for instance with line workers training – you have to ensure they understand the importance of what you are training them on, but at the same time, you cannot go too deep into the training, that would be either unnecessary or redundant.”
So what kind of training will continue to be the focus for the industry? The usual ones will continue to be important, according to Smith: Regulatory requirements for seafood, specific HACCP requirements for meat, HACCP training for employees directly responsible for food safety and quality, standards training; training for audits… For line workers, training also needs to cover specifics of their jobs, employee hygiene practices (for instance, why it’s important to wear gloves, hairnets etc.), and how to handle customer complaints.
Proposed rules under the Food Safety Modernization Act are having a positive impact on training needs, says Smith. “Everyone is now waiting for the final set of rules to be announced and implemented. A lot of good things are coming out of it, such focus on risk management and process control, understanding which products are high risk and low risk. FSMA has brought back HACCP for those industries that haven’t really had strict regulatory requirements for food safety – such as seafood, fresh produce, juice etc., or ready-to-eat products that may have escaped regulatory scrutiny in the past, such as fresh produce, and bakery products. Now the industry as a whole needs to focus on FSMA implementation, by companies helping its employees understand where the food safety risks are, how to manage them, document them, and mitigate them.
How can training help companies towards certification? When training leaders that are going to be implementing the food safety standards within their facility, it’s important to be comprehensive about understanding the specific standard. There is also the opportunity to focus on common non-conformances and say ‘this is where others have struggled and this is what you can do to avoid those non-conformances.’ Training can provide lessons, solutions and ideas to address common problems to addresses non-conformances before an audit. It can help drive continuous improvement, and help secure management commitment.
What are some of the common training themes that have resonated with Smith? He says that mostly employees bring up the issue of getting management to understand the need to focus on food safety training and procedures. “People who have attended my classes are usually struggling with getting management commitment. They are often told they need to be certified to a particular standard by end of the year, and so figure it out, but there’s no skin in the game from management. I help these trainees walk through the various steps that they can take to secure that commitment, how to talking their language in terms of more efficient production, and higher dollar savings etc.,” Smith describes.
Some challenges that he has observed in his years of training? Smith says that often times the trainers are great auditors, but poor trainers. “They will quote standards, and regulations. They are too black and white, and objective. However, training can have successful outcomes only when it’s practical and linked to real situations and ideas.”










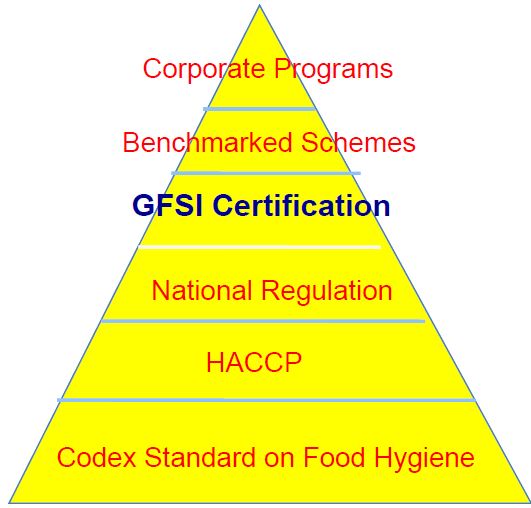

 At the very base of our efforts that are ensconced within these guidance on a sector by sector basis, are the international standards of science based within the Codex Standard on Food Hygiene. On top of that are Hazard Analysis and Critical Control Points or HACCP standards. Above HACCP are National Regulations, which includes FSMA in the U.S. and the Safe Food for Canadians Act in Canada. In Europe it’s something different, in Japan it’s something different, but all have iterative levels of science-based regulation in place to ensure the safest control of the food and management of the food. Above National Regulation is GFSI Certification.
At the very base of our efforts that are ensconced within these guidance on a sector by sector basis, are the international standards of science based within the Codex Standard on Food Hygiene. On top of that are Hazard Analysis and Critical Control Points or HACCP standards. Above HACCP are National Regulations, which includes FSMA in the U.S. and the Safe Food for Canadians Act in Canada. In Europe it’s something different, in Japan it’s something different, but all have iterative levels of science-based regulation in place to ensure the safest control of the food and management of the food. Above National Regulation is GFSI Certification.



