Recent food scandals around the world have generated strong public concerns about the safety of the foods being consumed. Severe threats to food safety exist at all stages of the supply chain in the form of physical, chemical and biological contaminants. The current pandemic has escalated the public’s concern about cross contamination between people and food products and packaging. To eliminate food risks, manufacturers need robust technologies that allow for reliable monitoring of key contaminants, while also facilitating compliance with the ISO 17025 standard to prove the technical competence of food testing laboratories.
Without effective data and process management, manufacturers risk erroneous information, compromised product quality and regulatory noncompliance. In this article, we discuss how implementing a LIMS platform enables food manufacturers to meet regulatory requirements and ensure consumer confidence in their products.
Safeguarding Food Quality to Meet Industry Standards
Food testing laboratories are continually updated about foodborne illnesses making headlines. In addition to bacterial contamination in perishable foods and ingredient adulteration for economic gains, chemical contamination is also on the rise due to increased pesticide use. Whether it is Salmonella-contaminated peanut butter or undeclared horsemeat inside beef, each food-related scandal is a strong reminder of the importance of safeguarding food quality.
Food safety requires both preventive activities as well as food quality testing against set quality standards. Establishing standardized systems that address both food safety and quality makes it easier for manufacturers to comply with regulatory requirements, ultimately ensuring the food is safe for public consumption.
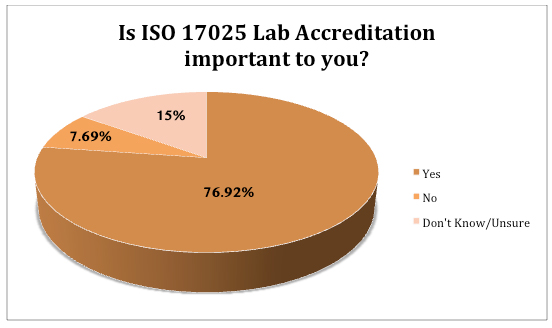
In response to food safety concerns, governing bodies have strengthened regulations. Food manufacturers are now required to ensure bacteria, drug residues and contaminant levels fall within published acceptable limits. In 2017, the ISO 17025 standard was updated to provide a risk-based approach, with an increased focus on information technology, such as the use of software systems and maintaining electronic records.
The FDA issued a notice that by February 2022, food testing, in certain circumstances, must be conducted in compliance with the ISO 17025 standard. This means that laboratories performing food safety testing will need to implement processes and systems to achieve and maintain compliance with the standard, confirming the competence, impartiality and consistent operation of the laboratory.
To meet the ISO 17025 standard, food testing laboratories will need a powerful LIMS platform that integrates into existing workflows and is built to drive and demonstrate compliance.
From Hazard Analysis to Record-Keeping: A Data-Led Approach
Incorporating LIMS into the entire workflow at a food manufacturing facility enables the standardization of processes across its laboratories. Laboratories can seamlessly integrate analytical and quality control workflows. Modern LIMS platforms provide out-of-the-box compliance options to set up food safety and quality control requirements as a preconfigured workflow.
The requirements set by the ISO 17025 standard build upon the critical points for food safety outlined in the Hazard Analysis and Critical Control Points (HACCP) methodology. HACCP, a risk-based safety management procedure, requires food manufacturers to identify, evaluate and address all risks associated with food safety.
The systematic HACCP approach involves seven core principles to control food safety hazards. Each of the following seven principles can be directly addressed using LIMS:
- Principle 1. Conduct a hazard analysis: Using current and previous data, food safety risks are thoroughly assessed.
- Principle 2. Determine the critical control points (CCPs): Each CCP can be entered into LIMS with contamination grades assigned.
- Principle 3. Establish critical limits: Based on each CCP specification, analytical critical limits can be set in LIMS.
- Principle 4. Establish monitoring procedures: By defining sampling schedules in LIMS and setting other parameters, such as frequency and data visualization, procedures can be closely monitored.
- Principle 5. Establish corrective actions: LIMS identifies and reports incidents to drive corrective action. It also enables traceability of contamination and maintains audit trails to review the process.
- Principle 6. Establish verification procedures: LIMS verifies procedures and preventive measures at the defined CCPs.
- Principle 7. Establish record-keeping and documentation procedures: All data, processes, instrument reports and user details remain secured in LIMS. This information can never be lost or misplaced.
As food manufacturers enforce the safety standards set by HACCP, the process can generate thousands of data points per day. The collected data is only as useful as the system that manages it. Having LIMS manage the laboratory data automates the flow of quality data and simplifies product release.
How LIMS Enable Clear Compliance and Optimal Control
Modern LIMS platforms are built to comply with ISO 17025. Preconfigured processes include instrument and equipment calibration and maintenance management, traceability, record-keeping, validation and reporting, and enable laboratories to achieve compliance, standardize workflows and streamline data management.
The workflow-based functionality in LIMS allows researchers to map laboratory processes, automate decisions and actions based on set criteria, and reduce user intervention. LIMS validate protocols and maintain traceable data records with a clear audit history to remain compliant. Data workflows in LIMS preserve data integrity and provide records, according to the ALCOA+ principles. This framework ensures the data is Attributable, Legible, Contemporaneous, Original and Accurate (ALCOA) as well as complete, consistent and enduring. While the FDA created ALCOA+ for pharmaceutical drug manufacturers, these same principles can be applied to food manufacturers.
Environmental monitoring and quality control (QC) samples can be managed using LIMS and associated with the final product. To plan environmental monitoring, CCPs can be set up in the LIMS for specific locations, such as plants, rooms and laboratories, and the related samples can then be added to the test schedule. Each sample entering the LIMS is associated with the CCP test limits defined in the specification.
Near real-time data visualization and reporting tools can simplify hazard analysis. Managers can display information in different formats to monitor critical points in a process, flag unexpected or out-of-trend numbers, and immediately take corrective action to mitigate the error, meeting the requirements of Principles 4 and 5 of HACCP. LIMS dashboards can be optimized by product and facility to provide visibility into the complete process.
Rules that control sampling procedures are preconfigured in the LIMS along with specific testing rules based on the supplier. If a process is trending out of control, the system will notify laboratory personnel before the product fails specification. If required, incidents can be raised in the LIMS software to track the investigation of the issue while key performance indicators are used to track the overall laboratory performance.
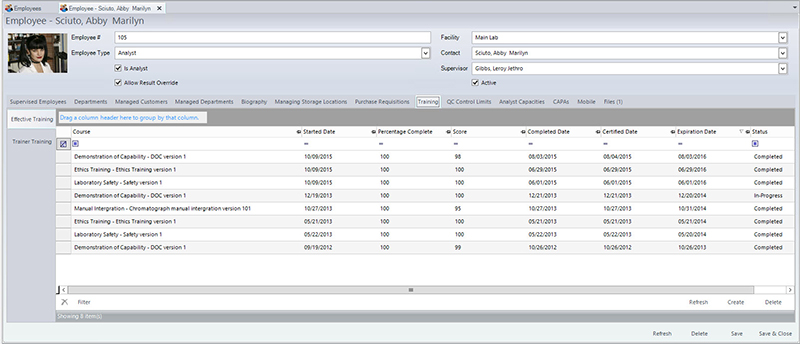
Tasks that were once performed manually, such as maintaining staff training records or equipment calibration schedules, can now be managed directly in LIMS. Using LIMS, analysts can manage instrument maintenance down to its individual component parts. System alerts also ensure timely recalibration and regular servicing to maintain compliance without system downtime or unplanned interruptions. The system can prevent users from executing tests without the proper training records or if the instrument is due for calibration or maintenance work. Operators can approve and sign documents electronically, maintaining a permanent record, according to Principle 7 of HACCP.
LIMS allow seamless collaboration between teams spread across different locations. For instance, users from any facility or even internationally can securely use system dashboards and generate reports. When final testing is complete, Certificates of Analysis (CoAs) can be autogenerated with final results and showing that the product met specifications. All activities in the system are tracked and stored in the audit trail.
With features designed to address the HACCP principles and meet the ISO 17025 compliance requirements, modern LIMS enable manufacturers to optimize workflows and maintain traceability from individual batches of raw materials all the way through to the finished product.
Conclusion
To maintain the highest food quality and safeguard consumer health, laboratories need reliable data management systems. By complying with the ISO 17025 standard before the upcoming mandate by the FDA, food testing laboratories can ensure data integrity and effective process management. LIMS platforms provide laboratories with integrated workflows, automated procedures and electronic record-keeping, making the whole process more efficient and productive.
With even the slightest oversight, food manufacturers not only risk product recalls and lost revenue, but also losing the consumers’ trust. By upholding data integrity, LIMS play an important role in ensuring food safety and quality.