Consumer preference for organic and “all natural” foods remains on the rise, according to market trend research and retailer sales.1,2 The Organic Trade Association (OTA) recorded $40 billion in U.S. organic food sales for 2015, stating that sales have nearly doubled since 2008.3 Pair this with $21 billion in sales for Q1 2016 for non-GMO labeled foods and $1.6 billion in 2015 gluten-free sales and, it is hard to ignore this thriving market sector, which seeks to support consumers in their quest for fresh, healthy and transparently-labeled foods.4,5
As a result of these trends, the industry is experiencing a surge in natural food and beverage start-up companies as well as the acquisition of organic and natural product companies by manufacturing giants such as Campbell Soup Co., Danone and General Mills, Inc. But in complex—and especially global—supply chains, achieving transparency comes with hurdles for verifying product claims such as “all-natural”, non-GMO, antibiotic-free, and other nutrient content or functional claims.
Organic and other natural food manufacturers are under increasing regulatory and consumer scrutiny for tracing claims back to the source for all ingredients. Failing to verify the authenticity or identity preservation (IP) status of materials, maintain chain of custody and ensure the accuracy of labels can have devastating consequences for a manufacturer, including regulatory action and consumer fraud class action law suits.6 It’s not just consumers demanding the “right to know” where food comes from, but manufacturers must also push this sentiment back through their supply chain to drive transparency for ensuring safety, brand protection and verifying product claims.
With the goal of meeting consumer demands for healthy food products, improved transparency in food production and clean labels, how can organic, non-GMO and natural food manufacturers stay ahead of the curve when it comes to ensuring that product claims provide the value consumers seek?
Consider the following tasks for achieving transparency in organic and natural product claims.
Analyze Your Ingredients for Risk
Get to know the pitfalls, which can affect the integrity of product claims. Many of these stem from cross contamination, authenticity or mislabeling issues for sourced materials. To prevent these pitfalls, analyze each ingredient for supply chain risks. Identifying potential risks, which may affect the integrity of claims creating liability for misbranding, is a critical step in achieving transparency.
For example, is there a potential for cross contamination from a non-organic source? This is a common risk where a supplier engages in the co-production of organic and non-organic materials. A lack of segregation and clear product identification during transportation, storage and processing activities can lead to commingling or cross-contamination, which affects material integrity and thus, any downstream product claims. Ensuring suppliers and the manufacturer have clear measures in place for segregation is an important consideration when determining risk.
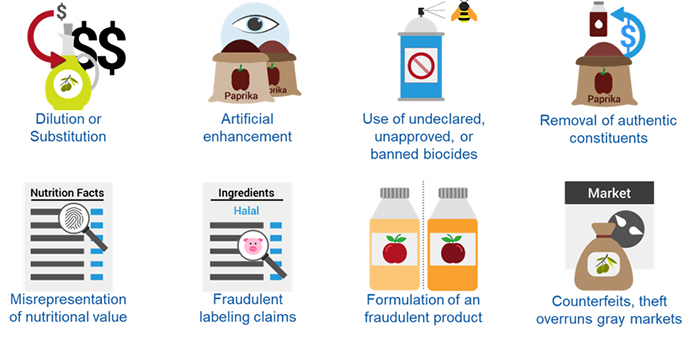
Or, consider adulteration from a non-authentic material, which can affect the integrity of the claim. Identifying vulnerabilities within the supply chain is necessary to reduce opportunities for perpetrating food fraud. Materials such as organic products and some natural ingredients are at greater risk for fraud where limited availability is an issue and/or the material is a high-value commodity or product. Mislabeling, counterfeit production or economically motivated adulteration, such as the substitution or dilution of ingredients in a sourced material, has a significant impact on downstream product claims.
Unverified packaging and labels are other sources of risk with the potential to affect the integrity of product claims. Ensure your supplier’s labeling practices include controls to verify the correct packaging and labels when producing IP materials or other ingredients with nutrient content or functional claims.
With a clear understanding of material risks, what attributes of an ingredient should be prioritized, tested and/or verified when considering the integrity of finished product claims?
Once material risks are analyzed, establish clear specifications for raw materials, which are agreed upon between the supplier and manufacturer. This serves as the basis for verifying material claims and subsequently, downstream product claims. Where specifications are in place, material verification may be performed through a variety methods including: testing, mass balance, COA review and audits. Verifying materials against agreed upon specifications not only supports due diligence in product claims but also brings manufacturers closer to their suppliers, steering us towards the next task.
Get to Know Your Suppliers
At the heart of food production transparency is the relationship a manufacturer has with its suppliers. Even the simplest of manufactured foods have a handful of ingredients, which are typically sourced through a global supply chain network. Due to the seasonality of produce or supply chain risks such as market fluctuations, business disruptions, natural disasters, or transportation failures; manufacturers can’t rely on a single supplier for the sourcing of a particular ingredient.
This leads to reliance on multiple suppliers, which may be geographically dispersed. Sourcing from multiple suppliers—especially when this occurs for multiple ingredients across multiple products—can create hurdles to relationship building for enhanced transparency due to time and resource constraints for acquiring first-hand knowledge of a supplier’s operation. Thus, proactive supply chain management, which enables a manufacturer to learn about the supplier’s history and operation, is essential for transparency.
This can be accomplished by establishing supplier approval criteria to provide a baseline for getting to know your supplier and establish minimum criteria for sourcing. Building upon this, is the use of approved suppliers to solidify the relationship and develop out a stable supply chain network. And finally, it is best practice to visit the supplier’s site to learn more about operational practices and the people responsible for ensuring material specifications and identity status are consistently achieved.
Apply Supply Chain Management Best Practices
Effective management of suppliers to prevent or reduce risks, which can lead to mislabeling and false claims, relies on the risk assessment conducted for materials and suppliers, applied controls (e.g., segregation) and verification that the supplier’s controls consistently ensure material integrity.
GFSI benchmarked schemes paved the way for enhanced supply chain management and risk mitigation when it comes to sourcing materials to ensure food safety and legal status. Some schemes additionally require controls and verification activities such as the validation of health claims or verification of nutrient content to provide a framework for helping manufacturers develop a system, which ensures product integrity. For food sold in the United States, a GFSI-based system is now reinforced by the FSMA Preventive Controls rule, which requires supply chain-applied controls to mitigate material risks along with additional controls to ensure that food is not adulterated or misbranded under the U.S. Food, Drug and Cosmetic (FD&C) Act.
It is important to note that while the FSMA Preventive Controls rule regulates most processors and manufacturers, organic raw agricultural commodities (RAC’s), dietary supplements and unprocessed meats are not covered by the rule as they are covered by other U.S. food regulations. Since these products may be included in organic and natural product formulations, manufacturers may want to consider applying a Preventive Controls methodology to their supply chain or pursue certification to a recognized food safety standard such as a GFSI benchmarked scheme where this is not already in place.
Simplify Your Supply Chain
Complex supply chains reduce visibility, add latency into monitoring, and increase opportunities for contamination or fraud.7,8
Simplifying your supply chain can take a variety of forms such as the sourcing of local or domestic materials.
Continue reading the article by clicking on page 2 below.