Implementation of FSMA will result in greater scrutiny of foreign material in food products at every stage of production, as well an entirely new pressure to locate and eliminate the source of contamination from the supply and production chain. Identifying foreign materials found in food products is the first step in determining their source, and therefore in determining how to prevent a given foreign material from being introduced into the product. For identification of small particles ranging from 1–1000 µm, microanalytical techniques are essential.
Examining and Isolating Foreign Material
Before the foreign material is prepared for analysis, the material is examined under a stereomicroscope. Ideally, isolation of the foreign particles from the host matrix and preparation of the foreign particles for microanalysis is performed in a cleanroom, which mitigates the introduction of environmental contamination not related to the initial contamination problem.
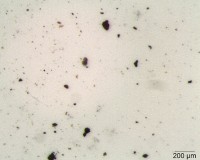
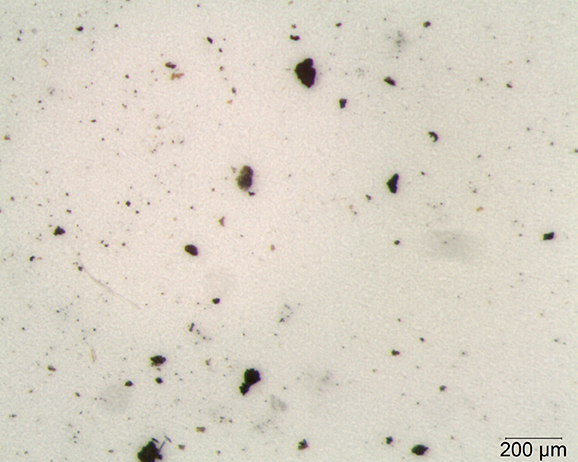
Under the stereoscope, the foreign material is isolated from the product matrix using a tungsten needle probe. It is photographed and the physical characteristics of the material (color, elasticity, magnetic properties, etc.) are observed and documented. Figure 1 shows particles filtered from a liquid product. In this case, the particles are approximately 100 μm and smaller. Most of the particles appear black to dark brown/orange in color. Some are brittle, while others are not. All are magnet responsive.
Only a few particles must be picked and prepared for analysis in this case because the particles are roughly similar. The coloration of the particles, along with their mechanical properties (magnetic, brittle, hard) indicate that the material is likely inorganic; scanning electron microscopy with energy dispersive X-ray microspectrometry (SEM-EDS) could be used to determine the elemental makeup of the material. The coloration and mechanical properties imply the source of the particles could be production machinery.
Identifying Inorganic Compounds with SEM-EDS
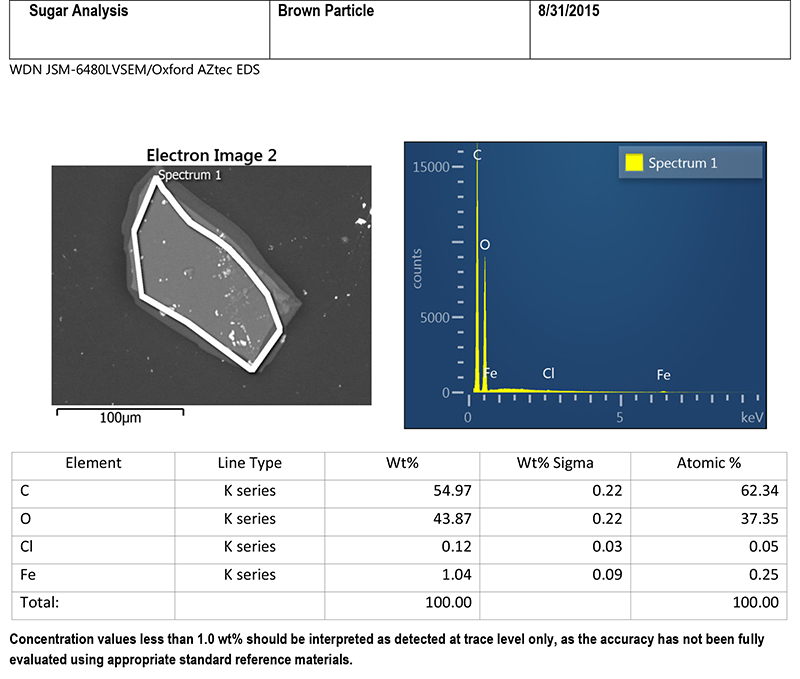
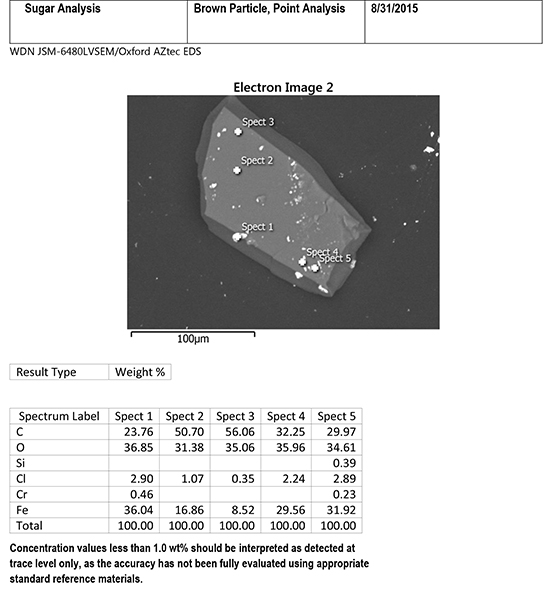
In a scanning electron microscope, a beam of electrons is scanned over the particle producing several signals, some of which are used for imaging, and some that are used for elemental analysis. For this discussion, the signals of interest for elemental analysis are X-rays. The energies of the X-rays are characteristic of the elements found in the sample. By counting these X-rays and arranging them according to their energies, a spectrum is produced, and elements in the sample can be identified and quantified.

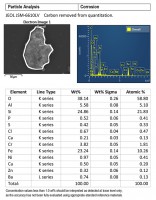
Figures 2 and 3 are SEM-EDS data from one of the brittle particles from the filter. The particle is steel corrosion (iron, chromium, and nickel), possibly with brass corrosion (copper and zinc) and some silicate material (elevated silicon and aluminum). Residues of corrosive agents (chlorine and sulfur) are present. The inclusions analyzed are 300 series stainless steel (see Figure 2). SEM-EDS data from one of the harder dark particles is shown in Figure 4. This particle is oxidized 300 series stainless steel, likely Type 316. 300 series stainless steels are not generally magnetic, but magnetism can be induced during wear processes.

Stainless steels, particularly Type 304 and Type 316 are common in food manufacturing environments. Pinpointing the source of these materials as contaminants can be frustrating due to the number of pieces of equipment made from these alloys. However, other metals are less common, such as Waukesha 88, a bismuth containing nickel-based alloy that is used in pump rotors and other moving parts because of its wear properties. Another less-common alloy is Type 321 stainless steel, a titanium stabilized stainless steel that is used in high temperature equipment where corrosion resistance is needed. Materials such as these are more easily traceable to their source, and therefore more easily repaired and thus eliminated as a source of foreign particles.
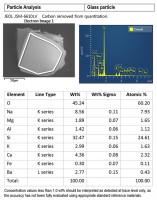
Other inorganic materials, such as glass, are also amenable to identification by SEM/EDS. SEM-EDS data from a glass particle is shown in Figure 5. Often, the glass can be identified as soda-lime glass or borosilicate glass. Soda-lime glass is commonly used for glass containers and bakeware; it is a mixture of oxides, mostly silicon dioxide, sodium oxide, and calcium oxide with smaller amounts of other oxide compounds. Borosilicate glass, commonly used in heat-resistant labware, contains silicon dioxide with a few weight percent boron trioxide, along with other oxide compounds; its composition results in a low coefficient of thermal expansion, and it is used in applications where its chemical and heat resistance are necessary. Identifying the glass type is helpful in determining the source of glass particles.
Identifying Organic Compounds using Fourier Transform Infrared Micro Spectroscopy (Micro-FTIR) Analysis
The SEM-EDS method cannot uniquely identify organic compounds, as it provides only elemental information—an EDS spectrum of organic material shows major carbon, and if it is degraded, oxygen. Protein will contain nitrogen as well.

FTIR analysis can identify most organic and a few inorganic materials. For small particles, micro-FTIR (an FTIR system with a microscope coupled to it) is used. Micro-FTIR analysis requires that the sample be thin enough to transmit light, since the system passes a beam of infrared radiation through the sample and records the frequencies at which the sample absorbs infrared radiation. The spectrum from a given material is unique, and even mixtures of materials can often be identified by comparison to known spectra from a reference library using an automated computer search.
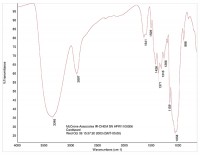
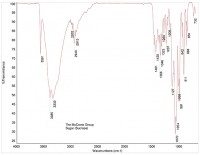
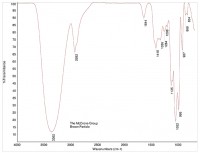
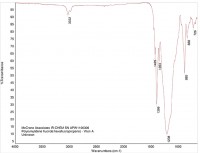
In this way, organic materials such as Viton O-rings can be identified (Figure 6 is a reference spectrum for Viton). Other organic material may be present in the product, such as cellulose (see Figure 7) or cardboard (see Figure 8). While these materials are not dangerous as small particles, they are not desirable in food products. When these kinds of things are found, tracing them to their source may be simple (as in the case of the O-ring material) or hard (cellulose can come from paper or cotton clothing, for example).
If the organic material found has inorganic fillers like titanium dioxide or silicon dioxide, then SEM-EDS can be used in concert with the micro-FTIR to refine the material description and simplify the process of identifying the source of the foreign material.
When used in tandem, SEM-EDS to identify inorganic materials and micro-FTIR to identify organic materials can be powerful tools for determining the origin of foreign particles. These microanalysis methods are essential tools for identifying and tracing the source of contaminant particles in food.