In the modern world, it’s often taken for granted that consumers can head to their local grocery store and fill their baskets with a broad range of meat, poultry, fish and dairy produce. Yet the plentiful availability of these products is possible, to a large extent, thanks to modern farming methods that rely on veterinary drugs to promote healthy animal growth, protect livestock from contracting diseases, and in some cases provide aesthetic qualities to food.
Despite the important role veterinary drugs play in farming and food production, usage must be carefully controlled, as their inappropriate administration can have adverse effects on animals, the environment and human health. A particular concern is the growing problem of antimicrobial resistance, which can be promoted in the environment by the overuse of some of these veterinary drugs.
As a result, the analysis of veterinary drugs forms an important part of routine food safety and quality control testing. However, the wide range of residue concentrations required to be quantified, along with the diverse sample matrices and chemical properties of multiple classes of veterinary drugs placed in a single analytical method, pose significant analytical challenges. The latest multi-residue, multi-class analytical workflow solutions using a generic sample preparation method and liquid chromatography tandem mass spectrometry (LC-MS/MS) are overcoming these issues to provide a robust, sensitive method for the extraction, detection, confirmation, and quantitation of veterinary drugs below their required maximum residue limits (MRLs).
Meeting the Needs of Veterinary Drug Analysis Workflows
Given the need to accurately and reliably quantify veterinary drugs in food, testing workflows must be both sensitive and operationally robust. Importantly, workflows must be amenable to a variety of different matrices, including meat, fish and dairy, and should be capable of screening for drug molecules with a broad range of physicochemical properties. The sample preparation protocols that are employed must minimize the loss of analytes and be sufficiently simple and cost effective to enable routine laboratory use. Additionally, the separation steps that are employed must be sufficiently rugged and should ideally be able to handle any analyte and matrix. Finally, the methods used to identify and quantify samples must be sufficiently selective and sensitive to detect and confirm drug molecules and their metabolites at trace levels.
Developing methods that can meet all of these criteria for a wide range of drug molecules and food matrices, while minimizing the potential for false positive and negative results, is not straightforward and has proven challenging for the industry. As a result, many analytical methodologies have emerged that are typically limited in scope to a limited number of residues or specific chemical classes, are labor intensive, and require extensive sample preparation and clean-up. Fortunately, ongoing advances in veterinary drug analysis workflows are helping to drive the adoption of standardized protocols that have universal applicability.
QuEChERS: Making Sample Preparation Quick, Easy and Reliable
Sample preparation is a key first step in veterinary drug analysis workflows, but its importance is often overlooked. Even with the most advanced downstream separation and detection technologies, workflows are liable to generate poor quantitative results without reliable residue extraction methods.Having robust sample preparation protocols is especially important given the heterogeneous nature of the sample matrix and the different physicochemical properties of the residues that must be extracted.
Traditional approaches, based on sample homogenization and multi-step solvent extraction procedures, were time-consuming and did not always produce consistent results. The loss of residues during sample grinding or through the formation of insoluble drug-matrix complexes would often impact the accuracy of measurements. Moreover, the need for labor-intensive sample cleanup steps, based on separation methods such as gel permeation chromatography, added additional complexity to workflows.
The widespread adoption of universal sample preparation protocols based on QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) methods has simplified the process of extracting veterinary drugs from matrix samples. These approaches have been specifically designed to be quick and easy to implement, and enable high extraction efficiency with a very broad range of chemical properties from a variety of matrices. As a result, QuEChERS has proven to be a very reliable means of preparing samples for veterinary drug analysis.
The universal suitability of the QuEChERS approach has reduced the complexity of sample preparation workflows to such an extent that many suppliers now offer kits containing pre-weighed reagents that can be used straight from the box. Moreover, because they only require small amounts of sample and solvent, and little in the way of equipment, these easy-to-use methods are helping laboratories minimize waste and make workflows more cost-effective.
Triple Quadrupole MS: Design Improvements Driving Exceptional Sensitivity
LC-MS/MS has rapidly established itself as the go-to technique for sensitive and reliable veterinary drug analysis, with set-ups based on ultra-high performance liquid chromatography (UHPLC) systems and triple quadrupole mass spectrometers proving to be particularly effective. With drug residues typically on the parts per billion scale, these systems have proven to be more than capable of delivering the level of performance that’s required when working with analytes that require low detection limits.
What’s more, recent advances in triple quadrupole mass spectrometer technologies are pushing the limits of quantitation even further. Improved instrument designs based on segmented quadrupoles, more powerful electron multipliers and enhanced ion transmission optics are enabling food analysis laboratories to achieve even greater levels of experimental sensitivity, mass accuracy, selectivity and precision. These performance improvements are allowing analysts to make more confident decisions around every sample.

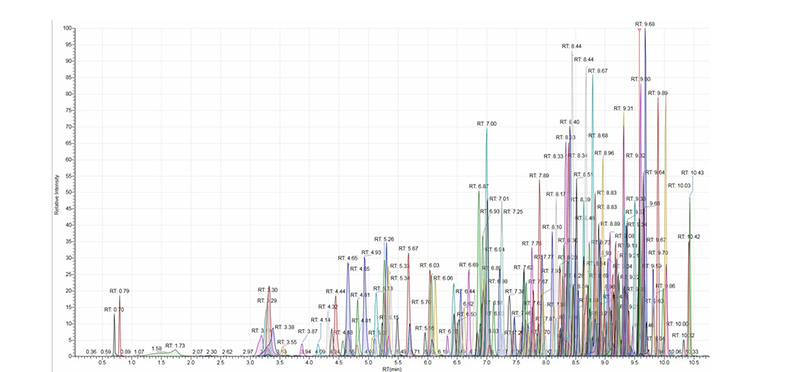
The capabilities of the latest generation of triple quadrupole LC-MS/MS systems for quantitative veterinary drug analysis were put to the test in a recent study. More than 170 veterinary drugs were added directly to a variety of homogenized matrices, including bovine muscle, milk, and salmon fillet using a QuEChERS sample preparation protocol to create a series of matrix extracted spikes (MES). The concentration of residues in the MES samples referenced a chosen screening target concentration (STC), which was typically one-third to one-quarter of the defined European Union MRL for each residue/matrix combination. Figure 1 presents the total extracted ion chromatogram for an MES sample of salmon fillet at the STC, obtained with a binary UHPLC system and a triple quadrupole mass spectrometer.
For each analyte, calibration curves were constructed using replicate measurements of each of the MES samples at seven concentrations ranging from one-fifth to five times that of the STC. Figure 2 highlights the calibration curve constructed for ethyl violet, a therapeutic dye used in aquaculture, in

the range 0.2 to 5.0 ng/g (STC = 1 ng/g). The calculated method detection limit of 0.03 ng/g for this compound in salmon fillet demonstrates confidence in the results well below the minimum required performance limit (MRPL).
LC-MS/MS: Leading the Way in Workflow Robustness
With potentially hundreds of samples to analyze every week, veterinary drug analysis workflows not only demand the highest levels of sensitivity, but also exceptional speed and robustness.
One way in which greater throughput can be achieved is by using shorter instrument dwell times, an experimental optimization that allows more compounds to be analyzed within a given timeframe during a chromatographic separation. Traditionally, the use of shorter dwell times would typically require sacrificing some measurement sensitivity. However, the latest advances in triple quadrupole instrument design are ensuring short dwell times no longer come at the expense of analytical performance.
Timed selected dreaction monitoring (SRM) is an effective strategy that allows analysts to overcome this challenge to achieve sensitivity with high throughput. Using timed SRM, data acquisition occurs within a short retention time window. This reduces the number of transitions that are monitored in parallel for each residue peak, while ensuring consistent quantitation even at low concentrations. Instrument control system software can automatically optimize the SRM conditions across the chromatographic run, maximizing operational efficiency with minimal need for manual input.
Instrument uptime is another factor that is of paramount importance for veterinary analysis workflows. With large workloads and tight turnaround times, regular instrument recalibration and frequent maintenance can be a major frustration for busy food testing laboratories. UHPLC is renowned for its operational robustness and suitability for fast-paced routine screening workflows, and the latest instruments are taking this reputation to an even higher level.

Figure 3 compares injections of bovine muscle extract at 3× STC over a 500-injection run that took place over a period of one week, obtained using the experimental set-up described earlier. Despite continuous operation over this extended period, the peak shape, intensity and retention time stability are maintained. These results further highlight the robustness of the LC-MS/MS system for routine veterinary drug testing.
Conclusion
Enforcing the responsible use of veterinary drugs in farming and food production depends upon comprehensive, sensitive, robust and reliable workflows capable of delivering quantitative results. Advances in sample preparation techniques and LC-MS/MS technologies are setting new standards when it comes to confident multi-residue veterinary drug analysis. From the development of reliable easy-to-use QuEChERS protocols, through to robust UHPLC separation methods and sensitive triple quadrupole mass spectrometers, improvements across the workflow are driving exceptional performance—whatever the matrix, whatever the residue.