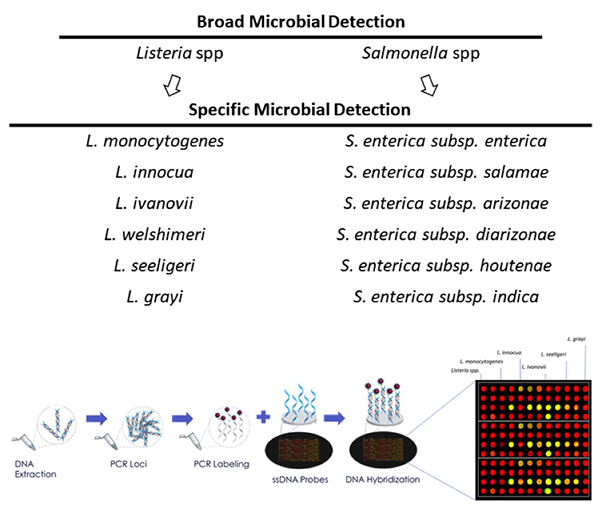
The microbiology lab will increasingly be understood as the gravitational center of big data in the food industry. Brands that understand how to leverage the data microbiology labs are producing in ever larger quantities will be in the best position to positively impact their bottom line—and even transform the lab from a cost center to a margin contributor.
The global rapid microbiology testing market continues to grow at a steady pace. The market is projected to reach $5.09 billion by 2023, up from $3.45 billion in 2018. Increased demand for food microbiology testing—and pathogen detection in particular—continues to drive the overall growth of this sector. The volume of food microbiology tests totaled 1.14 billion tests in 2016—up 15% from 2013. In 2018 that number is estimated to have risen to 1.3 billion tests, accounting for nearly half the overall volume of industrial microbiology tests performed worldwide.
The food industry is well aware that food safety testing programs are a necessary and worthwhile investment. Given the enormous human and financial costs of food recalls, a robust food safety testing system is the best insurance policy any food brand can buy.
We are going through a unique transition where food safety tests are evolving from binary tests to data engines that are capable of generating orders of magnitude of more information. This creates a unique opportunity where many applications for big data collected from routine pathogen testing can help go beyond stopping an outbreak. Paired with machine learning and other data platforms, these data have the opportunity to become valuable, actionable insights for the industry.
While some of these applications will have an impact on fundamental research, I expect that big data analytics and bioinformatics will have significant opportunity to push the utilities of these tests from being merely a diagnostic test to a vehicle for driving actions and offering recommendations. Two examples of such transformations include product development and environmental testing.
Food-Safety Testing Data and Product Development
Next-generation-sequencing (NGS) technologies demonstrate a great deal of potential for product development, particularly when it comes to better understanding shelf life and generating more accurate shelf-life estimates.
Storage conditions, packaging, pH, temperature, and water activity can influence food quality and shelf life among other factors. Shelf-life estimates, however, have traditionally been based on rudimentary statistical models incapable of accounting for the complexity of factors that impact food freshness, more specifically not being able to take into consideration the composition and quantity of all microbial communities present on any food sample. These limitations have long been recognized by food scientists and have led them to look for cost-effective alternatives.
By using NGS technologies, scientists can gain a more complete picture of the microbial composition of foods and how those microbial communities are influenced by intrinsic and extrinsic factors.
It’s unlikely that analyzing the microbiome of every food product or unit of product will ever be a cost-effective strategy. However, over time, as individual manufacturers and the industry as a whole analyze more and more samples and generate more data, we should be able to develop increasingly accurate predictive models. The data generation cost and logistics could be significantly streamlined if existing food safety tests evolve to broader vehicles that can create insights on both safety and quality indications of food product simultaneously. By comparing the observed (or expected) microbiome profile of a fresh product with the models we develop, we could greatly improve our estimates of a given product’s remaining shelf life.
This will open a number of new opportunities for food producers and consumers. Better shelf-life estimates will create efficiencies up and down the food supply chain. The impact on product development can hardly be underestimated. As we better understand the precise variables that impact food freshness for particular products, we can devise food production and packaging technologies that enhance food safety and food quality.
As our predictive models improve, an entire market for these models will emerge, much as it has in other industries that rely on machine learning models to draw predictive insights from big data.
Data Visualization for Environmental Monitoring
In the past one to two years, NGS technologies have matured to the point that they can now be leveraged for high-volume pathogen and environmental testing.
Just as it has in other industries, big data coupled with data visualization approaches can play a mainstream role in food safety and quality applications.
Data visualization techniques are not new to food safety programs and have proven particularly useful when analyzing the results of environmental testing. The full potential of data visualizations has yet to be realized, however. Visualizations can be used to better understand harborage sites, identifying patterns that need attention, and visualize how specific strains of a pathogen are migrating through a facility.
Some of this is happening in food production facilities already, but it’s important to note that visualizations are only as useful as the underlying data is accurate. That’s where technologies like NGS come in. NGS provides the option for deeper characterization of pathogenic microorganisms when needed (down to the strain). The depth of information from NGS platforms enables more reliable and detailed characterization of pathogenic strains compared to existing methods.
Beyond basic identification, there are other potential use cases for environmental mapping, including tracking pathogens as they move through the supply chain. It’s my prediction that as the food industry more broadly adopts NGS technologies that unify testing and bioinformatics in a single platform, data visualization techniques will rapidly advance, so long as we keep asking ourselves: What can the data teach us?
The Food Data Revolution and Market Consolidation
Unlike most PCR and immunoassay-based testing techniques, which in most cases can only generate binary answers, NGS platforms generate millions of data points for each sample for up to tens to hundreds of samples. As NGS technologies are adopted and the data we collect increases exponentially, the food safety system will become the data engine upon which new products and technologies are built.
Just as we have seen in any number of industries, companies with access to data and the means to make sense of it will be in the best position to capitalize on new revenue opportunities and economies of scale.
Companies that have adopted NGS technologies for food safety testing will have an obvious advantage in this emerging market. And they won’t have had to radically alter their business model to get there. They’ll be running the same robust programs they have long had in place, but collecting a much larger volume of data in doing so. Companies with a vision of how to best leverage this data will have the greatest edge.