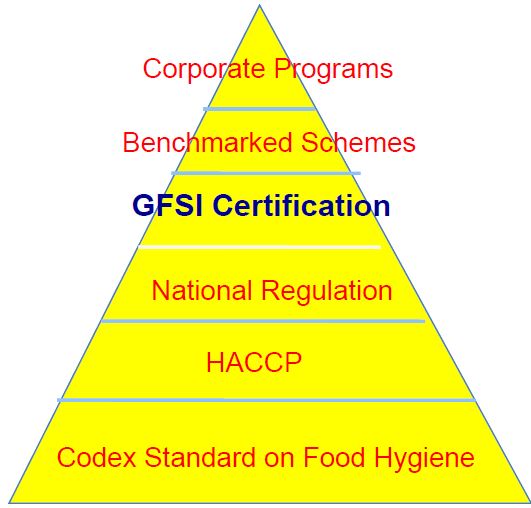
Introduction
The safety of our food supply is critical to public health, economic stability, and consumer confidence. The United States has developed a comprehensive regulatory framework for food safety that relies heavily on federal laws and oversight. This system involves multiple federal agencies, state and local governments, and numerous statutes designed to ensure food safety from farm to table. Private standards, such as those established by the Global Food Safety Initiative (GFSI), also play a role, but they are not a substitute for the regulatory power and public accountability inherent in U.S. food laws. This artilce argues that U.S. food laws are more effective than private standards and that businesses can achieve sufficient food safety by adhering to these laws without incurring additional costs associated with private standard implementation.
The Current U.S. Food Safety System
The U.S. food safety system is multifaceted, involving producers, processors, shippers, retailers, and consumers. The government, particularly through agencies like the FDA, USDA, EPA, and CDC, plays a critical role in establishing and enforcing food safety standards. These agencies work under numerous statutes and interagency agreements to ensure the safety of the food supply. The system, while complex and somewhat fragmented, is backed by legal enforcement, scientific research, and public accountability.
The Effectiveness of U.S. Food Safety Laws
The U.S. food safety regulatory system is extensive and multifaceted, involving numerous agencies with specialized roles. The Food and Drug Administration (FDA), the Food Safety and Inspection Service (FSIS) of the U.S. Department of Agriculture (USDA), the Environmental Protection Agency (EPA), and the National Marine Fisheries Service (NMFS) of the Department of Commerce all play crucial roles in ensuring food safety. These agencies operate under a framework of more than 35 statutes overseen by 28 Congressional committees, reflecting the complexity and thoroughness of the system.
The FDA, for instance, regulates domestic and imported foods, except for meat and poultry products, ensuring they are safe, sanitary, and properly labeled. The agency has regulatory authority over more than $1 trillion in products sold annually—about 25 cents of every dollar spent by consumers, including surveillance, risk assessment, research, inspection, and education. Similarly, the FSIS is responsible for ensuring the safety of meat and poultry products, employing roughly 7,400 inspectors to oversee 6,200 slaughtering and processing plants through continuous and daily inspections.
The statutory mandates for these agencies are comprehensive. For example, the FDA’s Center for Food Safety and Applied Nutrition (CFSAN) enforces tolerances for pesticide residues, approves food additives, and ensures that food processing plants adhere to safety standards. The FSIS, by law, must conduct carcass-by-carcass inspection during slaughter, ensuring that meat and poultry products are safe for consumption. USDA) announced in 2021 that government will invest more than US$4 billion to improve the food system.
Government Enforcement and Public Accountability
U.S. food laws are enforced by federal agencies with the authority to conduct inspections, enforce regulations, and impose penalties for non-compliance. This legal backing ensures a high level of adherence to food safety standards. For example, the FDA and USDA conduct regular inspections and surveillance to ensure compliance with food safety laws. This system of oversight provides a level of public accountability that is essential for maintaining consumer trust and preventing foodborne illnesses.
Scientific Basis and Comprehensive Coverage
U.S. food laws are grounded in scientific research and are regularly updated to reflect the latest food safety information. These laws cover a wide range of food products and hazards, including microbiological contaminants, pesticides, and additives. This comprehensive approach ensures that all potential risks are addressed, providing a higher level of protection for consumers.
Economic and Consumer Protection
Effective food safety regulations prevent large-scale foodborne illness outbreaks, reducing healthcare costs and economic losses. By ensuring that food products meet safety standards, the government protects consumers from the dangers of contaminated or unsafe food. This protection is crucial for maintaining public health and economic stability.
Limitations of Private Standards
While private standards like those established by GFSI can complement public regulations, they are not a substitute for the robust framework provided by U.S. food laws. Private standards are voluntary and lack the enforcement power of government regulations. They often require third-party audits, which can be costly and lead to inconsistent implementation due to variability in auditor competency and interpretation of standards.
Cost and Market Pressure
Certification and compliance with private standards can be expensive, particularly for small and medium-sized enterprises. These costs can create barriers to market entry and place unnecessary financial burdens on businesses. Moreover, companies may feel pressured to adopt private standards to remain competitive, regardless of whether these standards offer additional safety benefits over existing regulations.
Transparency and Accountability
Private standards are enforced through independent third-party audits, which may lack the transparency and public accountability of government inspections. The results of these audits are not always made public, and there is less oversight to ensure that auditors maintain high standards of integrity and competence.
Limited Scope and Inequality
Private standards may not cover all aspects of food safety, especially those specific to local contexts or emerging threats. Additionally, smaller companies and producers in developing countries may face significant barriers to accessing certification due to costs and resource constraints, leading to inequality in market access and potentially restricting trade opportunities.
The Case for Relying on U.S. Food Laws
Given the comprehensive and enforceable nature of U.S. food laws, businesses can achieve sufficient food safety by adhering to these regulations without incurring additional costs associated with private standards. The legal framework provided by U.S. food laws is designed to protect consumers and ensure food safety across the entire supply chain. By focusing on compliance with these laws, businesses can avoid the complexities and expenses of private certification while still maintaining high standards of food safety.
HACCP Systems and FSMA
The implementation of Hazard Analysis Critical Control Point (HACCP) systems and the Food Safety Modernization Act (FSMA) has strengthened the U.S. food safety framework. HACCP systems focus on preventing hazards at critical points in the production process, while FSMA emphasizes proactive measures to prevent food safety issues. These regulatory approaches are widely recognized as effective means of ensuring food safety and can be more cost-effective than adhering to multiple private standards.
Comparison with Private Standards
Private food safety schemes, like those endorsed by GFSI, offer standardized protocols for food safety management across global supply chains. These schemes are often seen as more stringent and market-driven, providing a competitive edge and reassurance to consumers and business partners. However, these private standards come at a cost. Businesses must pay for certification, ongoing compliance audits, and the implementation of specific protocols that may overlap with existing regulatory requirements.
Comparison of U.S. Food Laws vs. GFSI Standards
Pros and Cons of GFSI Standards
|
GFSI Standards |
|
|
Pros |
Cons |
|
|
Pros and Cons of U.S. Food law
|
U.S. Food Laws |
|
|
Pros |
Cons |
| 1. Government Enforcement: U.S. food laws are enforced by federal agencies like the FDA and USDA, providing a robust and legally backed framework for food safety.
2. Comprehensive Coverage: These laws cover a wide range of food products and hazards, including pesticides, additives, and microbiological contaminants. 3. Public Accountability: Government agencies are accountable to the public, with transparent operations and public reporting of food safety issues. 4. Standardization: U.S. food laws provide standardized regulations across the country, ensuring uniform food safety practices. 5. Scientific Basis: The laws are based on scientific research and updated with the latest food safety information. 6. Interagency Coordination: Multiple agencies collaborate, providing a multi-faceted approach to food safety. 7. Consumer Protection: The primary goal is to protect consumers from foodborne illnesses and unsafe food products. 8. Economic Impact: Government-regulated food safety can prevent large-scale foodborne illness outbreaks, reducing healthcare costs and economic losses. 9. Legally Binding: Compliance is mandatory, with legal consequences for non-compliance, ensuring high levels of adherence. 10. Educational Resources: Government agencies provide extensive educational resources and support to food businesses to help them comply with regulations. |
|
Conclusion
While both U.S. food laws and GFSI standards have their strengths and weaknesses, their effectiveness depends on the specific context and needs of the food industry stakeholders. U.S. food laws provide a legally enforceable and standardized framework, ensuring a high level of consumer protection and public accountability. On the other hand, GFSI standards offer flexibility, global recognition, and industry-driven innovation, though they come with significant costs and may lack the enforcement power of government regulations.
The U.S. food safety system, despite its complexity and fragmentation, provides a robust and effective framework for ensuring the safety of the food supply. Federal regulations enforced by agencies like the FDA and USDA offer comprehensive coverage, scientific grounding, and legal enforcement, which are critical for protecting public health and consumer confidence. While private standards can play a supportive role, they cannot match the effectiveness and public accountability of government regulations. By adhering to U.S. food laws, businesses can achieve high standards of food safety without incurring the additional costs and complexities associated with private certification.
For optimal food safety, a balanced approach that leverages the strengths of both systems may be the most effective solution. This hybrid strategy would combine the stringent, enforceable standards of U.S. food laws with the flexibility and global perspective of private standards like GFSI, ensuring comprehensive protection for consumers while promoting innovation and international trade.
Further reading:
- (2023, July 13). FSMA Final Rule on Accredited Third-Party Certification. Retrieved July 18, 2024, from US Food and Drug Administration: https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-accredited-third-party-certification
- Foulis, D. (2024, Feb 14). Four benefits of gaining GFSI certification. Retrieved July 18, 2024, from Ideagen: https://www.ideagen.com/thought-leadership/blog/four-benefits-gfsi-certification
- (2011, March). Enhancing Food Safety Through Third Party Certifications. Retrieved July 18, 2024, from Mygfsi.com: https://mygfsi.com/wp-content/uploads/2019/09/Third-Party-Certification-GFSI-White-Paper.pdf
- Kaylegian, K. (2021, April 13). The Food Safety Modernization Act (FSMA). Retrieved July 18, 2024, from PennState Extension: https://extension.psu.edu/the-food-safety-modernization-act-fsma
- Loucks, S. (2024, May 31). Why Should My Business Become GFSI Certified? Retrieved July 18, 2024, from FoodReady: https://foodready.ai/blog/why-should-my-business-become-gfsi-certified/
- Scott, J. (2023, Nov 8). FDA FSMA: Providing value beyond compliance. Retrieved July 18, 2024, from IBM: https://www.ibm.com/blog/fda-fsma-providing-value-beyond-compliance/
- Ng S, Shao S, Ling N. Food safety risk-assessment systems utilized by China, Australia/New Zealand, Canada, and the United States. J Food Sci. 2022 Nov;87(11):4780-4795. doi: 10.1111/1750-3841.16334. Epub 2022 Oct 26. PMID: 36285586; PMCID: PMC9828015.
- Halabi, Sam. 2016. The Battle between Public and Private Food Safety Standards. Yale Journal of Regulations. https://www.yalejreg.com/nc/the-battle-between-public-and-private-food-safety-standards/ (access on 6/29/2024)
- Soon JM, Baines RN. Public and Private Food Safety Standards: Facilitating or Frustrating Fresh Produce Growers? Laws. 2013; 2(1):1-19. https://doi.org/10.3390/laws2010001
- National Research Council (US) Committee on the Review of Food and Drug Administration’s Role in Ensuring Safe Food; Wallace RB, Oria M, editors. Enhancing Food Safety: The Role of the Food and Drug Administration. Washington (DC): National Academies Press (US); 2010. 2, The Food Safety System: Context and Current Status. Available from: https://www.ncbi.nlm.nih.gov/books/NBK220410/
- Institute of Medicine (US) and National Research Council (US) Committee to Ensure Safe Food from Production to Consumption. Ensuring Safe Food: From Production to Consumption. Washington (DC): National Academies Press (US); 1998. 2, The Current US Food Safety System. Available from: https://www.ncbi.nlm.nih.gov/books/NBK209121/