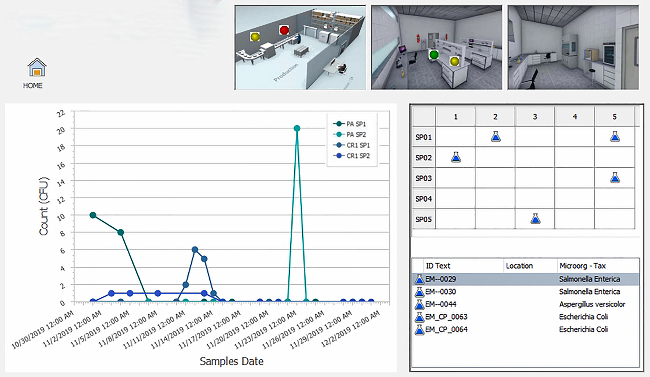
The COVID-19 pandemic heightened the urgency for food brands to adopt technology solutions that support remote management of environmental monitoring programs (EMPs) as they strive to provide safe products to customers. While digital transformation has progressed within the food safety industry, food and beverage manufacturers often have lower profitability as compared to other manufacturing industries, such as pharmaceutical and high-tech equipment, which can lead to smaller IT spend.1 Many companies still rely on manual processes for environmental monitoring and reporting, which are prone to error, fail to provide organizations with visibility into all of their facilities and limit the ability to quickly take corrective actions.
Despite growing recognition of the value of automating testing, diagnostics, corrective actions and analytic workflows to prevent contamination issues in food production environments, barriers to adoption persist. One key obstacle is the recurring mindset that food safety is a necessary compliance cost. Instead, we need to recognize that EMP workflow automation can create real business value. While the downside of food safety issues is easy to quantify, organizations still struggle to understand the upside, such as positive contributions to productivity and a stronger bottom-line achieved by automating certain food safety processes.
To understand how organizations are using workflow automation and analytics to drive quantifiable business ROI, a two-year study that included interviews and anonymized data collection with food safety, operations, and executive leadership at 34 food organizations was conducted.
The respondents represent more than 120 facilities using advanced EMP workflow automation and analytics. Based on the interviews and the shared experience of food organization leaders, two key examples emerged that demonstrate the ROI of EMP automation.
Improved Production Performance
According to those interviewed, one of the primary benefits of EMP automation (and driver of ROI) is minimizing production disruptions. A temporary conveyor shutdown, unplanned cleaning, or extensive investigatory testing can add up to an astounding 500 hours annually at a multi-facility organization, and cost on average $20,000 to $30,000 per hour.2 So, it’s obvious that eliminating costly disruptions and downtime has a direct impact on ROI from this perspective.
But organizations with systems where information collected through the EMP is highly accessible have another advantage. They are able to take corrective actions to reduce production impacts very quickly. In some cases, even before a disruption happens.
By automatically feeding EMP data into an analytics program, organizations can rapidly detect the root cause of issues and implement corrective actions BEFORE issues cause production delays or shutdowns.
In one example, over the course of several months, a large dairy company with manual EMP processes automated its food safety workflows, improved efficiencies, reduced pathogen positives and improved its bottom line. At the start of the study, the company increased systematic pathogen testing schedules to identify where issues existed and understand the effectiveness of current sanitation efforts. With improved access to data on testing, test types and correlated sanitation procedures, the company was able to implement a revamped remediation program with more effective corrective action steps.
Ultimately, the automated workflows and analytics led to reduced positive results and more efficient EMP operations for the company as compared to the “crisis-mode” approach of the past. The associated costs of waste, rework, delayed production starts, and downtime caused by food safety issues were significantly reduced as illustrated in Figure 1.
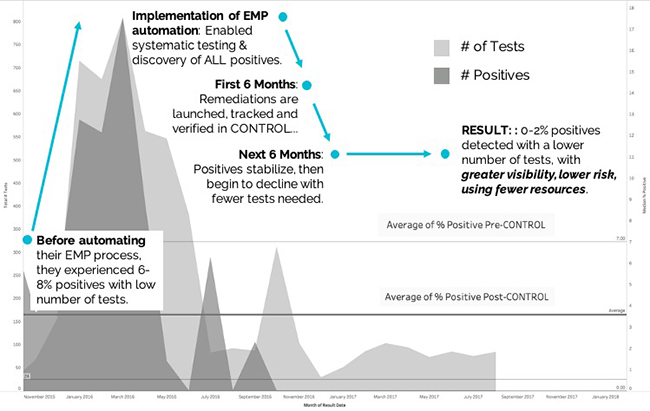
Quantifying the ROI of Production Performance Improvements
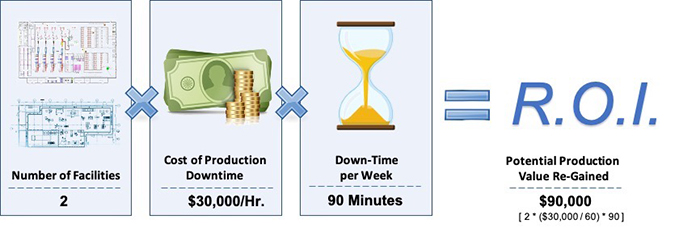
The financial impact of reducing production downtime by just 90 minutes per week can be dramatic when looked at by cumulative results over multiple weeks. In fact, eliminating just a few delayed starts or unplanned re-cleaning can have significant financial gains.
Figure 2 shows the business impact of gaining 90 minutes of production up-time per week by automating food safety operations. For the purposes of this analysis, the “sample organization” depicted operates two facilities where there are assumptions that down-time equates to a cost value of $30,000 per hour, and that both plants experience an average of 90 minutes of downtime per week that can be re-gained.
Reduced Food Waste
The second key insight uncovered in the two-year study was the impact that automating the EMP process had on waste. An estimated 30–40% of all food produced in the United States is wasted, and preventable food safety and quality issues account for a substantial portion of this waste.3
A key challenge shared by study participants was detecting food safety issues early enough to avoid wasting an entire production run. Clearly, the later in a processing or manufacturing run that issues are discovered, the greater the potential waste. To limit this, organizations needed near real-time visibility into relevant food safety and EMP data.
By automating EMP workflows, they solved this issue and created value. By tracking and analyzing data in near real time, production teams were able to keep up with ever-moving production schedules. They could define rules to trigger the system to automatically analyze diagnostic results data and alert stakeholders to outliers. Impacted food product could be quickly identified and quarantined when needed before an entire production run was wasted.
Companies included in the study realized substantial benefits from the increased efficiencies in their testing program. According to a food safety quality assurance manager at a large U.S. protein manufacturer, “Our environmental monitoring program has reached new heights in terms of accuracy, communication, visibility and efficiency. Manual, time-intensive tasks have been automated and optimized, such as the ability to search individual sample or submittal IDs, locate them quickly and make any necessary changes.”
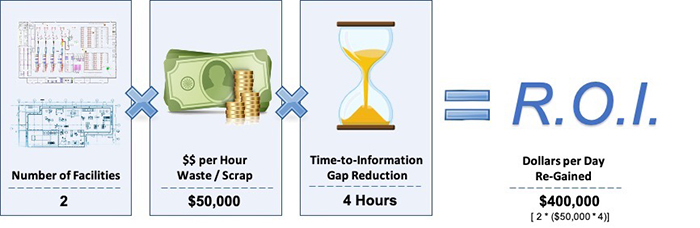
Quantifying the ROI of Food Waste Reductions
Figure 3 shows how measuring the business impact of gaining back just 10% of scrapped food per week. For the purposes of this analysis, the “sample organization” depicted operates two facilities where there are 500 lbs. of finished product scrapped each week, and the value per pound of finished product is valued at a cost of $1 per pound.
Conclusion
Automating EMP workflows decreases the time required to receive and analyze critical EMP data, helping food manufacturers achieve significant improvements in production performance, waste reduction and overall testing efficiency. By using these same ROI calculations, food brands can better illustrate how improved food safety processes can build value, and help leaders see food safety as a brand imperative rather than a cost center. As food organizations progress through each stage of digital transformation, studies like this can show real-world examples of business challenges and how other organizations uncovered value in adoption of new technologies and tools.
References
- CSIMarket, Inc. (2021). Total Market Profitability.
- Senkbeil, T. (2014). Built to Last: Maintaining Reliability and Uptime of Critical Connected Systems in Industrial Settings. Anixter.
- USDA. Food Waste FAQs.