
Food quality management is complex and the overall economics of quality are often underestimated.

Food quality management is complex and the overall economics of quality are often underestimated.
SafetyChain’s FSQA Tech Talk conversation continues next week with a discussion on why cloud and mobile technologies are becoming a game changer for food safety and quality assurance (FSQA).
As consumers demand to know the “who, what, when, where and how” of products they purchase, companies must focus on bringing honesty to the table to build trust.
The biggest risk faced by the food and beverage industry could be the supply chain itself.
All methods are not equal, and companies must understand the testing methods used on a Certificate of Analysis.
Achieving complete traceability is a must to combating seafood fraud. How is industry getting there?
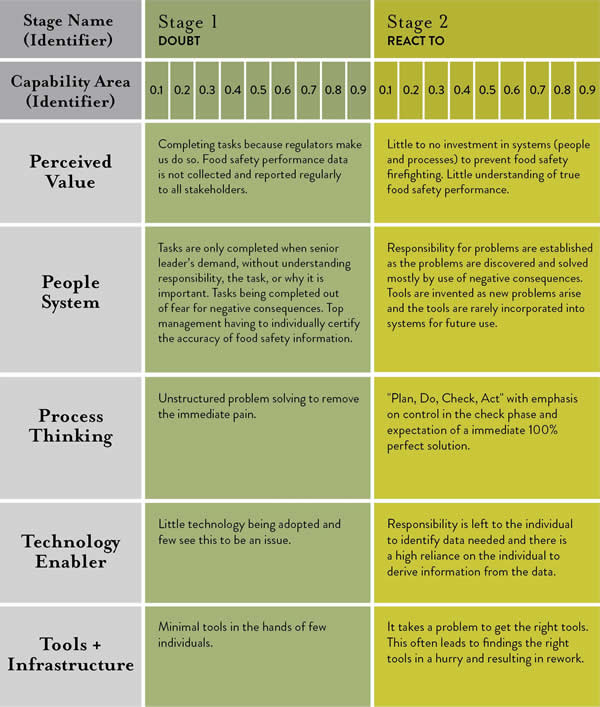
A renewed recognition of the importance of individual employee behavior within food processing and manufacturing organizations is shining a spotlight on awareness and accountability, but a standardized measure of food safety culture must be defined.
What’s the best way to collect supplier documentation? You might read that sentence and think there is no best way…and you would probably be correct. There really is no best way to gather documentation other than sending a representative out to a supplier’s facility for an audit and document gathering. But we simply don’t have enough personnel to go that route.

Whether it’s determining exactly which rules apply to you, migrating from HACCP to HARPC, or preparing for and dealing with increasing volumes of data and documents, here’s a list of the most critical FSMA challenges. Expert’s advice? Start preparing now for compliance.

FSQA enabling technologies can have a great impact on transforming and harmonizing food safety and quality assurance, mitigating risk and improving ability to meet operational KPIs, says SafetyChain Software’s Barbara Levin.