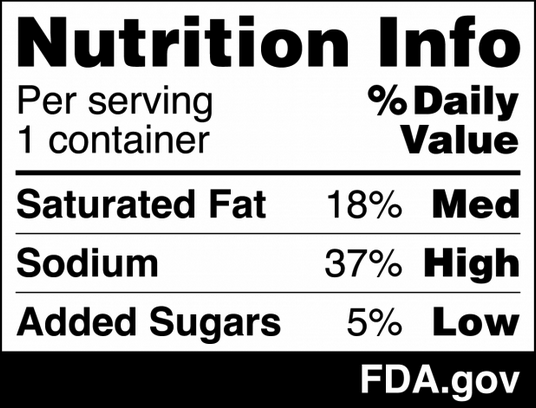
Supply chain stability has a great deal of influence over food safety and security across global markets. When food networks experience disruptions, the consequences affect production, distribution, storage conditions, and consumer well-being. Recent events, such as the COVID-19 pandemic, have demonstrated the urgency of developing adaptable food supply systems that maintain strict safety standards under pressure.
Modern food supply chains must balance operational excellence with strategic planning to respond to market shifts and evolving safety requirements. An ideal approach integrates vendor partnerships, technological solutions, and risk management practices to create robust networks that protect business value and public health. Successful food supply networks blend time-tested operational practices with smart innovation, maintaining quality standards through every market shift and challenge.
Streamlining Operations for Efficiency
Food supply networks depend on seamless coordination between multiple moving parts, from initial production to final delivery. Each step in the process — from cold storage management to transportation scheduling — requires precise timing and careful quality control. Optimizing these operations demands attention to two critical areas: building strong vendor partnerships and integrating smart technology solutions.
These core elements create reliable, efficient systems that maintain food safety while reducing operational costs. Success begins with cultivating strong partnerships throughout the vendor network and amplifying these relationships through strategic technology adoption. Striking this balance requires careful attention to supplier relationships and smart implementation of digital monitoring tools.
Building Strong Vendor Networks
Strong vendor relationships start with clear performance standards and consistent communication practices. Regular quality assessments and collaborative planning sessions help create lasting partnerships built on mutual success. These relationships become especially valuable during supply chain challenges when quick responses and flexible solutions matter most.
Effective vendor invoice processing reduces costs through automated systems and standardized procedures. This streamlined approach eliminates common error sources while freeing staff to prioritize strategic improvements. Well-managed vendor documentation also supports compliance efforts by maintaining clear records of all transactions and quality verifications.
Technology Integration for Safety and Efficiency
Advanced digital systems monitor food safety throughout storage and transportation, offering precise control over environmental conditions and product tracking. Modern IoT-driven sensor networks provide continuous updates on temperature, humidity, and other critical factors that affect food quality. These systems also utilize AI technology to analyze and formulate rapid responses to potential issues before they affect product safety.
Digital platforms also improve communication across the supply chain, connecting vendors, transporters, and facility managers through unified data systems. Real-time updates and automated alerts help maintain product integrity while reducing waste. Supply chain managers use this precise, real-time data to reinforce both immediate decisions and strategic planning.
Risk Mitigation and Flexibility in the Supply Chain
Food distribution networks face persistent challenges from multiple sources. Seasonal storms disrupt transportation routes, equipment failures compromise cold storage systems, and sudden demand spikes strain production capacity. Each type of disruption presents unique challenges to food safety and quality control, requiring specific strategies and response protocols.
Effective risk management combines two essential capabilities: systematic vulnerability assessment and operational flexibility. Organizations must develop methods to spot potential problems before affecting product quality. They then need adaptable systems that can quickly adjust to changing conditions without compromising safety standards.
Identifying and Addressing Vulnerabilities
Effective risk prevention begins with regular assessment of potential weak points. Transportation delays, equipment malfunctions, and storage complications can all threaten product integrity. Maintaining food safety during disruptions requires systematic monitoring and clear response protocols. Organizations particularly benefit from detailed contingency plans that anticipate various scenarios and outline specific actions for each situation.
Quality control teams must stay alert to subtle indicators that might signal developing problems. This vigilance includes monitoring supplier performance metrics, tracking delivery patterns, and analyzing temperature control data. Early detection of potential issues allows swift intervention before minor concerns escalate into significant disruptions.
Building Adaptable Systems
Flexible distribution networks accommodate sudden changes without sacrificing safety standards or operational efficiency. This adaptability stems from strategic redundancy in critical areas, including backup supplier relationships and alternative transportation routes. Cross-trained staff members provide additional flexibility, allowing quick reallocation of resources when specific areas need extra support.
Clear communication channels prove essential during periods of adjustment. Team members at every level need accurate, current information about changing conditions and modified procedures. Staff training drills sharpen emergency response skills and build team confidence. Well-prepared personnel protect food quality standards while smoothly executing needed process adjustments.
Branding and Consumer Trust in Food Safety
Consumer confidence grows from consistent safety practices paired with open communication. Food producers and distributors sharing detailed information about quality control measures build lasting market relationships. Today’s consumers look beyond basic safety claims — they want to understand specific handling procedures, storage protocols, and quality certifications. This heightened interest in food safety creates opportunities for meaningful dialogue with customers about quality assurance practices.
Meeting these expectations takes a coordinated approach that spans internal operations and external communications. Organizations can build consumer trust by creating clear, accessible messages about safety standards and fostering active participation in safety practices across all stakeholder groups. From employee training programs to consumer education initiatives, each element of safety communication plays a vital role in building and maintaining market confidence.
Communicating Safety Commitments
Clear messaging about food safety practices builds credibility with consumers and retail partners. Documentation of safety protocols, quality certifications, and handling procedures demonstrates dedication to product quality. Promotional products highlight your brand values while educating consumers about specific safety measures, from temperature monitoring to contamination prevention. If possible, tailor your materials to match the relationship in question.
Safety communication involves more than standard product packaging and labels. Websites, social media, and print materials collaborate to share detailed quality control processes and safety innovations. Using multiple communication methods helps consumers find accurate safety information wherever they look. Generally speaking, it’s better to have more information available than you need than to be too opaque.
Engaging Stakeholders in Safety Culture
Food safety excellence starts in warehouses and continues through every delivery route. Quality control teams conduct hands-on training sessions, from temperature monitoring to contamination prevention, to simulate real-world challenges. Staff members contribute valuable insights through structured feedback programs, often identifying practical improvements to daily operations. Employee recognition programs celebrate exceptional safety practices, spurring healthy competition and innovative approaches to quality control.
Safety education has far-reaching effects throughout the distribution network. Distribution centers conduct specialized training sessions for retail partners, teaching proper handling techniques for various food categories. Store staff learn optimal storage methods for different products and then pass this knowledge to customers through clear guidelines and practical tips. Local food safety workshops bring together warehouse teams, retail staff, and consumers, creating collaborative learning environments.
Final Thoughts
Strong food supply networks emerge from careful attention to three core elements: operational precision, risk readiness, and stakeholder engagement. Organizations that excel at vendor management while integrating advanced monitoring systems create reliable distribution channels that withstand market pressures. Clear safety protocols and quick-response capabilities maintain food quality through unexpected challenges.
Safety-focused communication strengthens every link in the food supply chain. Informed employees can spot potential issues early, trained vendors can follow precise handling procedures, and educated consumers can make smarter storage decisions. When each participant understands their role in maintaining food safety, the entire system benefits through consistent quality, reduced waste, and increased market confidence.