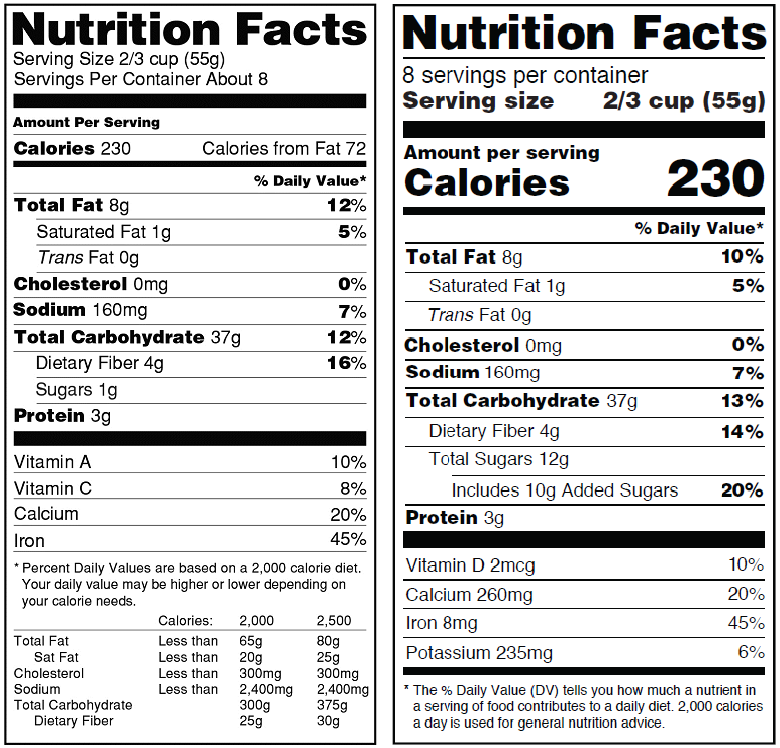
Menu Labeling
Another labeling issue pending before FDA relates to calorie disclosure on restaurant menus. In May 2017, the agency announced that it was delaying the compliance date for menu labeling requirements by a year, from May 5, 2017 to May 7, 2018. FDA explained that the delay would allow industry to identify opportunities to reduce costs and enhance flexibility of the requirements.
In general, convenience stores and grocers supported the delay because of the scope of the food establishments covered under the menu labeling requirements. However, the National Restaurant Association has long supported the rule because it would preempt a number of different requirements on the state and local level.
Frustrated over the delay, two consumer groups – the Center for Science in the Public Interest (CSPI) and National Consumers League – filed a lawsuit claiming that the delay would not assist industry in avoiding compliance costs and therefore, did not represent a good cause for the delay. For its part, FDA filed a motion to dismiss the case, arguing that the consumer groups lacked standing because they had not demonstrated how they were harmed by the delay.
Temporary Truce
FDA and the two groups recently filed a joint motion agreeing to pause litigation on the issue. As part of this agreement, FDA promised to confirm by the end of 2017 the compliance date of May 7, 2018 for the rule, or publish the industry compliance guidance by the end of 2017. If FDA fails to meet these obligations, litigation could resume.
Outlook
This temporary truce likely will prevent state and local governments from proceeding with their own menu labeling efforts, which were preempted by the federal law. Recently, New York City had threatened to begin enforcing its own rules, but reached an agreement with FDA on the May 7, 2018 compliance date. While it was already unlikely that other cities would move forward in light of the NYC-FDA agreement, the temporary truce between the consumer groups and FDA virtually assures it.
Allergens
Undeclared allergens on food labels have been the leading cause of U.S. food recalls for several years now, and it’s a problem that has long vexed the industry, especially given how preventable labeling errors are compared to other threats. According to the Center for Disease Control and Prevention (CDC), over 50 million Americans suffer from food allergies annually.
To address this problem, the Food Marketing Institute (FMI) Foundation recently announced that it is awarding the Food Allergy Research and Resource Program (FARRP) at the University of Nebraska – Lincoln’s Food Science and Technology Department a $20,000 grant to identify root-cause labeling errors. The research also will recommend best-practice procedures for manufacturers, suppliers and retailers in order to reduce undeclared allergen recalls.
In its press release announcing the grant, the FMI Foundation explained that, by focusing on how primary root-cause errors occur and identifying appropriate preventive measures, food allergen recalls can be reduced, thus creating better protection for food allergic consumers, reduce food waste, and decrease economic burden for suppliers, manufacturers and retailers.
The Food Allergy Research and Resource Program intends to present preliminary outcomes of their research at a conference in November 2017, and ultimately submit a paper for peer-reviewed publication.
Outlook
Because this effort is being supported by FMI, a large trade association representing the food retail industry, the recommendations for best-practices that emanate from this research have the potential of being implemented industry-wide on a voluntary basis. Given the likely absence of any legislative or regulatory activity that would be perceived as imposing additional burdens on industry, efforts such as this that are supported by industry and result in voluntary best practice guidelines certainly are worth monitoring.
Simplifying Date Labels
The Consumer Goods Forum, a network of approximately 400 large food and consumer goods companies from around the world announced an initiative recently that aims to simplify date labels worldwide. The group, which includes large companies such as Walmart, Kellogg, Campbell Soup and Amazon among others, is urging retailers and food producers to simplify date labels to reduce consumer confusion that has resulted in 1.3 billion tons of food being lost or wasted per year globally.
This effort corresponds with one announced earlier this year by the Grocery Manufacturers Association (GMA) and the Food Marketing Institute (FMI), which called for the use of two common phrases: ‘Best If Used By’ and ‘Use By.’
While the primary goal of this effort is to reduce food waste, attempts to simplify date labels have food safety implications as well. The term ‘Best if Used By’ is meant to inform consumers of the quality of a food product; the quality may not be optimal, but it conveys that it is still safe to consume. The ‘Use By’ date applies to highly perishable products, and is meant to inform the consumer that these products should be consumed by the date listed on the package and disposed of after the ‘use by’ date.
Outlook
Much like the effort to reduce labeling errors for allergens, this industry-supported effort to simplify date labels is expected to resonate and be applied widely on a voluntary basis. Food waste is becoming a higher profile issue, is receiving increased attention from policy makers, and could be addressed in the 2018 farm bill. Efforts by industry to address this issue voluntarily represent an opportunity to avoid or minimize any attempts to impose additional requirements through legislation or regulatory action.
Conclusion
The rulemaking process underway on labeling, along with the industry initiatives, reflects a strong desire by consumers to know more about the foods they purchase. This has been an ongoing trend that is likely to continue.