Forma Foods, a startup company incubated at Tecnologico de Monterrey, the top Mexican university in engineering and technology according to the QS World Ranking 2024, is leveraging cutting-edge 3D printing technologies to produce plant-based meat that not only looks and tastes like real meat, but also promotes a more sustainable and ethical approach to future food.
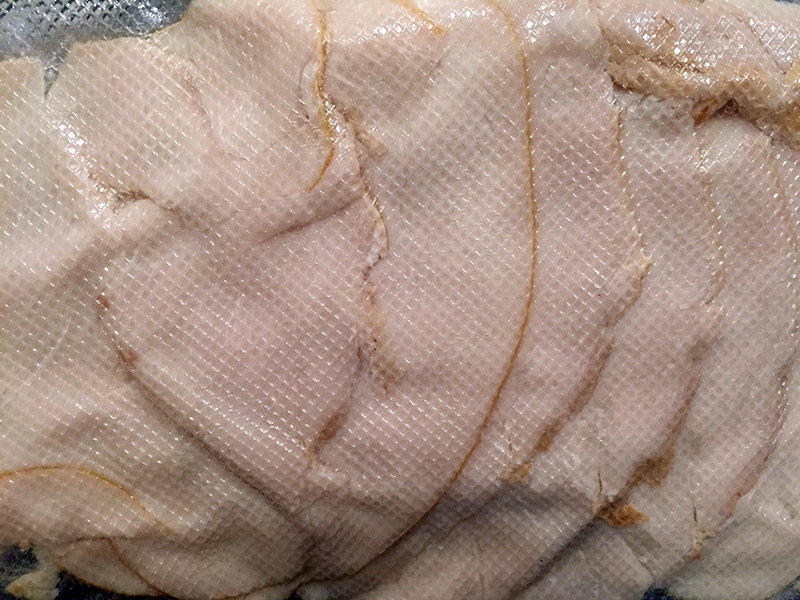
Lab-developed meat from Forma Foods emulates the texture, consistency, and flavor of traditional “carne asada”, offering a viable and attractive alternative for meat lovers and vegan consumers.
To this end, Grissel Trujillo de Santiago and Mario Moises Alvarez, researchers and academics from the School of Engineering and Sciences at Tec de Monterrey, experts in tissue engineering, and co-founders of the company, are applying a technique that both have invented and perfected: chaotic printing.
“These chaotic flows have nothing to do with disorder or turbulence, they produce microstructures in a fast and mathematically predictable way; they generate very fine thin layers that mimic the architecture of animal tissues,” explains Trujillo, Chief Scientific Officer (CSO) at Forma Foods.
Forma Foods not only mimics skeletal muscle tissue, but also fat and connective tissue, using pea protein to simulate muscle tissue, an oriental prebiotic fiber for connective tissue, and coconut oil for fat tissue.
The team has created products very similar to arrachera and carne al pastor, staples in dishes representative of Mexican culture. “Eating an arrachera taco is not the same as eating a sausage or ground beef taco,” Trujillo says, emphasizing how their product authentically integrates into Mexican cuisine.