Q&A Part I: Hiring and Training, Understanding FSMA Remain Big Industry ChallengesIn part two of Food Safety Tech’s Q&A with TraceGains, Anthony Arocha (customer success consultant), Rajan Gupta (vice president of customer success), and Jason Ulrich (customer success manager) explain the factors at play surrounding the lack of supplier documentation in the food industry.
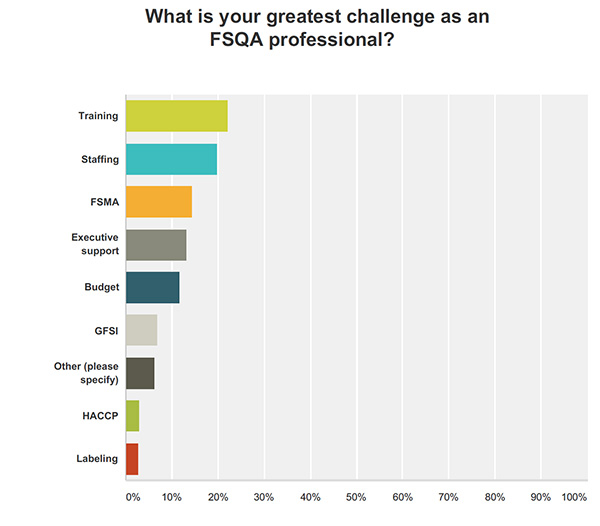
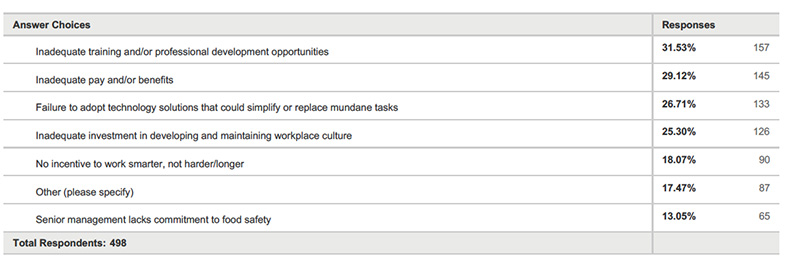
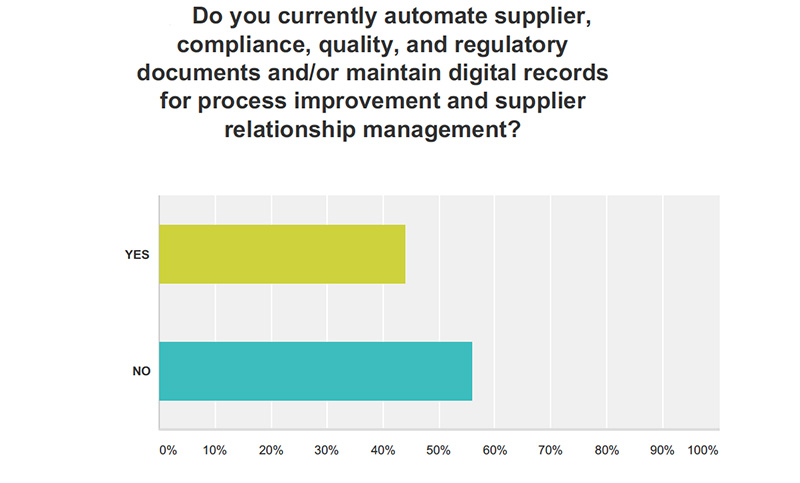
Food Safety Tech: Just 44% of respondents said they automate supplier documents. What information can you glean from this? Why aren’t more companies automating?
Arocha: Companies understand that using technology is essential to manage the increasing demands on accurate food safety documentation and verification. For many companies, it is likely to be just a timing and resource issue as to why they have not yet adopted automation—timing as in they have not yet reached the pain threshold required to justify the new cost to implement and to have a resource to support or focus on it. As companies grow and new budgets get created, it is just a matter of time before they will have to include automation help if they have not already.
Gupta: I believe lack of internal respect for QA and thus lack of education and funding are key contributors to this area. Most of the quality staff is stuck doing daily activities with limited time to explore options to make their processes better. Lack of empowerment to make business process changes is also a large factor in not adopting technology. Marc states that the companies have silos as indicated by the transparency gains from technology—while that is true, the root cause of this may be that the various groups within an organization have never really paid attention to FSQA areas and thus never envisioned having access to information that can help the organization proactively manage risk and increase food safety awareness.
Ulrich: This is all about people money, and time. The industry as a whole doesn’t have enough in quality departments. The lack of qualified individuals available in QA departments has always been an issue. The money is usually used to improve production and other departments except quality. That leaves the limited resources in the department with very little time to review and implement new processes or software.
FST: Regarding supplier documentation management, where are companies falling short?
Arocha: Supplier document management is not easy. You are at the mercy of your vendors. I think the biggest issue is trying to do everything too fast versus having a risk-based approach and focusing on the top priority items first. Build on success. If you try to do too much too fast, it is hard to pick out the success stories easily and can become overwhelming.
Gupta: Anthony is right but he is also stating the obvious problem – “mercy of vendors”. We believe that technology such as TraceGains Network can improve efficiency greatly in sharing documentation and risk-based data, but lack of education and rapid acceptance within the industry of new approaches hinders innovation and limits already stretched resources to take shortcuts that may not be the best course of action long-term.
Ulrich: In addition to what Marc, Anthony and Raj stated most are afraid to challenge the supplier. There is a fear of making them angry or asking for too much.