2018 and 2019 were the years of the “blockchain buzz”. As we enter the new decade, we can expect a stronger focus on how technology and data advances will generate more actionable use for the food industry. Food Safety Tech has highlighted many perspectives from subject matter experts in the industry, and 2020 will be no different. Our first Q&A of the year features Sasan Amini, CEO of Clear Labs, as he shares his thoughts on tech improvements and the continued rise consumer expectations for transparency.
Food Safety Tech: As we look to the year ahead, where do you see artificial intelligence, machine learning and blockchain advancing in the food industry?
Sasan Amini: AI, ML, and blockchain are making headway in the food industry through advances in supply chain management, food sorting and anomaly detection, and tracing the origin of foodborne outbreaks. On the regulatory side, FDA’s focus on its New Era of Smarter Food Safety will most likely catalyze the adoption of the above mentioned technologies. On the private side, a few of the companies leading the charge on these advancements are IBM and Google, working in partnership with food manufacturers and retailers across the world.
Along those same lines, another area that we expect to grow is the use of AI and ML in tandem with robotics—and the value of new troves of data that they collect, analyze and distribute. For example, robotics for the use of environmental monitoring of potential contaminants, sorting techniques and sterilization are valuable because they ensure that end products have been through thorough testing—and they give us even more information about the lifecycle of that food than ever before.
At the end of the day, data is only valuable when you can transform it into actionable insights in real-time with real-world applications, and we expect to see more and more of this type of data usage in the year ahead.
FST: Where do you think food safety testing technologies will stand out? What advancements can the industry expect?
Amini: In 2020, technology is going to begin to connect itself along the entire supply chain, bringing together disparate pieces and equipping supply chain professionals with action-oriented data. From testing advances that improve speed, accuracy and depth of information to modular software solutions to promote transparency, the food safety industry is finally finding its footing in a data-driven sea of technological and regulatory advances.
Right now, legacy testing solutions are limited in their ability to lead food safety and quality professionals to the source of problems, providing insights on tracking recurring issues, hence having a faster response time, and being able to anticipate problems before they occur based on a more data heavy and objective risk assessment tools. This leaves the industry in a reactive position for managing and controlling their pathogen problems.
Availability of higher resolution food safety technologies that provide deeper and more accurate information and puts them in context for food safety and quality professionals provides the food industry a unique opportunity to resolve the incidents in a timely fashion with higher rigour and confidence. This is very in-line with the “Smarter Tools and Approaches” that FDA described in their new approach to food safety.
FST: How are evolving consumer preferences changing how food companies must do business from a strategic as well as transparency perspective?
Amini: Consumers are continuing to get savvier about what’s in their food and where it comes from. Research suggests that about one in five U.S. adults believe they are food allergic, while only 1 in 20 are estimated to have physician-diagnosed food allergies. This discrepancy is important for food companies to consider when making decisions about transparency into their products. Although the research on food allergies continues to evolve, what’s important to note today is that consumers want to know the details. Radical transparency can be a differentiator in a competitive market, especially for consumers looking for answers to improve their health and nutrition.
Consumers are also increasingly interested in personalization, due in part to the rise in new digital health and testing companies looking to deliver on the promise of personalized nutrition and wellness. Again, more transparency will be key.
FST: Additional comments are welcome.
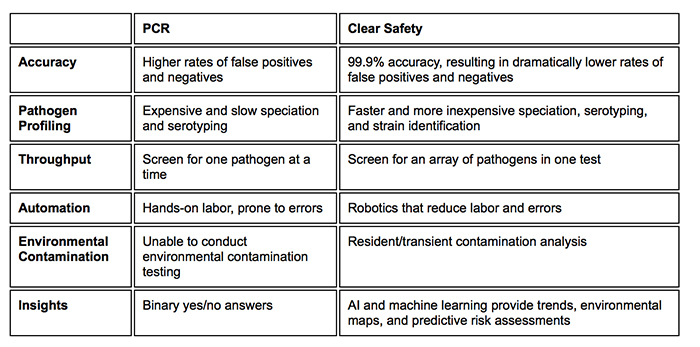
Amini: Looking ahead, we expect that smaller, multi-use, and hyper-efficient tools with reduced physical footprints will gain market share. NGS is a great example of this, as it allows any lab to gather millions of data points about a single sample without needing to run it multiple times. It moves beyond the binary yes-no response of traditional testing, and lets you get much more done, with far less. Such wealth of information not only increases the confidence about the result, but can also be mined to generate more actionable insights for interventions and root cause analysis.
This “multi-tool” will be driven by a combination of advanced software, robotics, and testing capabilities, creating a food safety system that is entirely connected, driven by data, and powerfully accurate.