The Internet of Things (IoT) is the concept that everything will one day be connected, similar to when computers became networked and connected with the internet. A sensor in a walk-in freezer is now smart enough to communicate directly with the smartphone in your pocket and a computer at the office, all in real-time. This is what IoT is all about, bringing more information to our fingertips in order to make faster, more informed decisions.
These new technologies are beginning to intersect and create new solutions to old problems, such as periodically monitoring the temperature of equipment in a restaurant or the trailer of a refrigerated truck. Savvy operators who understand changing food safety regulatory demands are driving the adoption of these technologies that ease the transition towards ongoing compliance. Food safety technology is changing, and what follows are a few of the driving forces.
Smartphones, Tablets and Cloud Computing Create Ready-made Environment
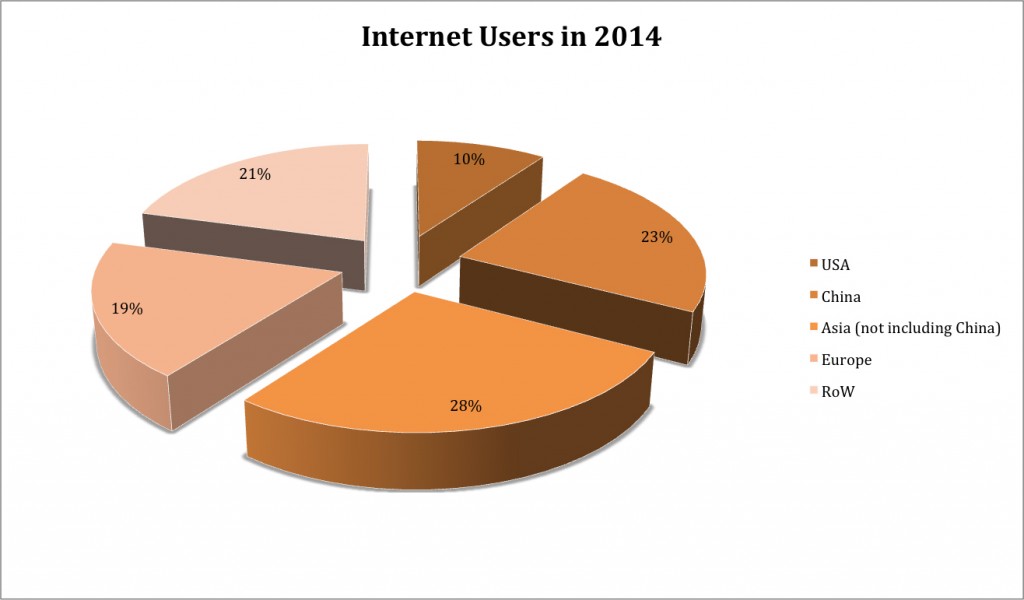
Apple launched the first iPhone in 2007 and within six years, 50% of the U.S. population was using a smartphone and/or tablet. Another market event that helped create the foundation for IoT was the growth of the “cloud” model where organizations could “rent” hardware, software and data storage. When coupled with new affordable wireless networking capabilities (WiFi, Bluetooth) and expanded cellular coverage at decreased cost rates for data, it became economically viable for nearly any size company operating in the foodservice industry to collect, store and access data.
Over the course of the last decade, we’ve become more comfortable living in a connected world and, as the technology has matured, businesses started to look at how smart devices could be used to improve operational efficiency and outdated food safety protocols. Instead of manually checking equipment temperatures, wireless sensors are now connecting refrigerators and other temperature controlled environments to the cloud. Any operator with a smartphone is now able to view these temperatures (or receive alerts) in real-time to ensure equipment and product temperatures meet company standards and local regulatory requirements.
Heightened Diligence by Oversight Agencies, Increased Consumer Activism and Brand Protection Concern
The responsibility for food safety spans both national (FDA/USDA/CDC) and local (state and county health department) organizations. FSMA has widened these responsibilities across the cold chain. With limited resources, operators are being asked to adopt new regulations and do their part to ensure the integrity of the product that is being stored and/or transported.
In addition, consumers have become increasingly self-aware regarding various food-related issues, including oversight and traceability (i.e. labeling, processing, etc.). This same general trend can be seen where consumers are now expecting ongoing food safety inspections and access to inspection results online. This puts more pressure on operators to ensure guidelines are met and inspections are passed.
Finally, restaurants are becoming more proactive in protecting their brand. The idea of keeping any incidents limited to the awareness of only the few that were involved is a thing of the past. Forward-thinking restaurants realize that social media has changed the landscape, and what was once a single-store minor infraction can now cause franchise-wide problems. Additionally, food safety is just good business. Restaurants have moved beyond following procedures as a necessary hurdle to now actively following and implementing best practices and policies in order to achieve operational efficiency and elevate their brand reputation.
IoT the Enabler of a Data-driven Business
Simply put, the internet has reshaped all businesses, so why not restaurants and the cold chain? With the availability of “ready-made tech”, sensors can connect to front-of-house and back-of-house environments to monitor temperature (frozen, refrigerated, ambient, hot-holding) in all types of equipment (walk-in refrigerators and freezers, under-counter coolers, showcase units and sandwich lines) to continuously and wirelessly monitor temperature and send alerts if the proper temperature is not maintained.
Data gathering can also be extended to incorporate digital task management capabilities to replace traditional Hazard Analysis and Critical Control Points (HACCP) manual logbooks and simplify daily restaurant tasks. Organizations can streamline manual operational checklists and provide insight to managers on how well their teams are adhering to restaurant guidelines.
Restaurants now have an important tool to address the two sides of food safety—prevention and traceability. Additionally, through capturing larger data sets, restaurants can move from anecdotal guesswork to implementing data-based best practices. The ingredients are now in place for restaurants to offer the highest levels of food safety and quality that the industry has ever enjoyed.