Food safety is one of the biggest health concerns worldwide. The World Health Organization estimates that about one in 10 people contract an illness from contaminated food, with more than 400,000 dying each year as a result. Food contamination leads to illness, death, lost productivity, and wasted money.
Fortunately, technology to improve food safety continues to advance. The shift moves toward automation and system monitoring to improve accuracy and decrease the time that workers must attend to routine tasks. With the use of these developing technologies, commercial and industrial organizations can help to prevent foodborne illness and provide greater transparency to the consumer.
Smart Devices
The Internet of Things (IoT) provides several opportunities to improve food safety in commercial and industrial spaces. The premise behind IoT is that each device or appliance can connect to a larger hub, providing information about the contents or function of the equipment in real-time. These tools can improve food safety by performing safety checks, recording data like food temperatures, and allowing centralized monitoring of the entire system. In kitchens, restaurants and food processing plants with fewer employees, the ability to get information at a touch can simplify routine tasks and improve accuracy.
Sensors
Advanced sensor technology improves old technology by centralizing data collection and providing real-time updates. Sensors work by assessing factors like temperature or humidity within a controlled space. Older systems required workers to visually inspect the temperatures and adjust as needed. The latest models provide integrated data with the capacity to send immediate alerts when the conditions fall below safe levels. The technology minimizes food waste by prompting quick action, while giving operators the ability to confirm that the food always remains at safe levels.
3D Printing
Testing materials can be expensive, proprietary, and difficult to fit to every application, which highlights the benefits of 3D printing. 3D printing relies on the slow accumulation of material into a specific 3D design. The broad availability of designs allows organizations to contract out 3D printing of testing tools or bring the entire production in-house. The technology is revolutionizing various industries for its ability to provide custom products at a fraction of the cost of traditional manufacturing, often for much less investment.
Automated Sanitation
Automated sanitation systems improve the speed of cleaning and decrease the risk of human error. UV light systems gained notoriety for their ability to dramatically reduce the spread of pathogens like SARS CoV-2, creating benefits for commercial and industrial applications. UV and ozone sanitation improve results over liquid disinfectants and provide better results for surfaces that need to be sterilized.
The automation of these and other sanitation systems significantly cut down on the time workers must spend cleaning up. Industrial automation solutions minimize the risk of contamination, as well as shortening equipment downtime.
Blockchain
Blockchain is best known for its use in cryptocurrency, but the premise offers potential to decrease the effects of food contamination. The United States Food and Drug Administration notes that traceability presents the biggest problem for handling outbreaks of listeria or E. coli. The United Nations Development Programme argues that blockchain’s decentralized organization allows for the accurate presentation of information that cannot be misrepresented or deleted. This technology can provide detailed tracking of a food’s journey from farm to table to reduce time and money spent determining the source of a contaminated food.
DNA Barcoding
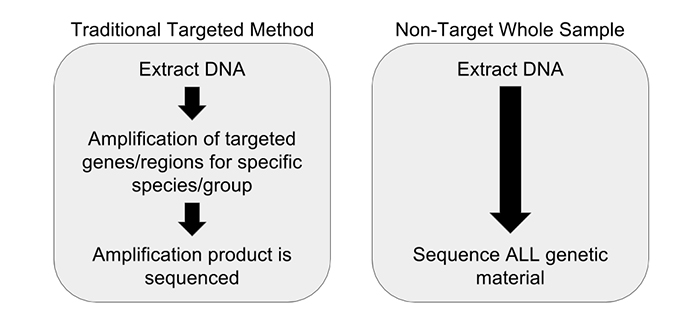
Preventing foodborne illness and the spread of pathogens involves greater effort to inspect during every step of the food’s journey. DNA barcoding presents interesting opportunities to quickly evaluate foods to classify their species and identify the presence of foreign bodies. The method highlights parts of the genome that identify a specific species, creating a barcode of sorts that can be used to verify the species. This level of detail in inspection can increase the accuracy of labeling, as well as providing a way to track the origin at a molecular level.
Automated Inspections
Human inspection can fail, especially for workers who may be operating in understaffed conditions. The increase in automated systems provides a viable solution. Visual systems can inspect each item for signs of damage or faulty production, immediately removing them from possible use. The latest technology can identify faults that are beyond human observation, increasing the accuracy of the equipment and minimizing the use of defective products.
Artificial Intelligence
Artificial intelligence (AI) has the potential to revolutionize all of these systems and more. AI uses the collection and processing of data to identify trends and highlight risks within a contained environment. The system can handle automated checks, maintenance, and other tasks that were once performed by humans. AI can use data to predict failures of systems or the likelihood of contamination, so that workers can address them in advance. With access to real-time data, AI can also provide immediate alerts to changes in food preparation or storage, to minimize food loss and the growth of foodborne illness.
Handling food in any capacity requires attention to food safety to minimize illness and death among the most vulnerable populations. In the interest of increasing food safety, researchers and manufacturers are investing in technology to automate food safety tasks such as sanitation and tracking food temperatures. While some technologies represent the vanguard of the food industry, others are gaining relevance worldwide. Exploring these technologies helps food safety professionals to evaluate the best systems to protect consumers throughout the use of their commercial and industrial spaces.
Sources
World Health Organization (WHO). (2022, May 19). Food safety. Who.int; World Health Organization: WHO. https://www.who.int/news-room/fact-sheets/detail/food-safety
Brous, P., Janssen, M., & Herder, P. (2019). The dual effects of the Internet of Things (IoT): A systematic review of the benefits and risks of IoT adoption by organizations. International Journal of Information Management, 51(1), 101952. https://doi.org/10.1016/j.ijinfomgt.2019.05.008
Pangarkar, T. (2024, May 7). 3D Printing Statistics 2024 By New Technology, Filaments, Types. Market Scoop. https://scoop.market.us/3d-printing-statistics/
US EPA, O. (2021, January 14). Disinfecting Surfaces with UV Light to Reduce Exposure to SARS-CoV-2. Www.epa.gov. https://www.epa.gov/emergency-response-research/disinfecting-surfaces-uv-light-reduce-exposure-sars-cov-2
NEW ERA OF SMARTER FOOD SAFETY FDA’s Blueprint for the Future. (n.d.). https://www.fda.gov/media/139868/download
Blockchain for Agri-Food Traceability | United Nations Development Programme. (n.d.). UNDP. https://www.undp.org/publications/blockchain-agri-food-traceability
DNA Barcoding – an overview | ScienceDirect Topics. (n.d.). Www.sciencedirect.com. https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/dna-barcoding