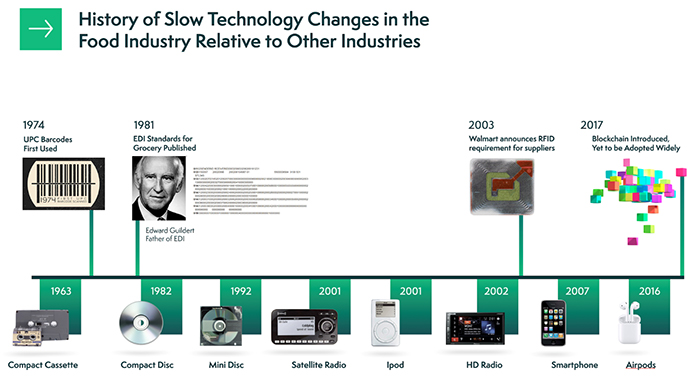
The theme of better traceability and more transparency is a theme that will only grow stronger in the food industry. Just last week we heard FDA Deputy Commissioner for Food Policy and Response Frank Yiannas talk about the agency’s recently proposed FSMA rule on food traceability during the 2020 Food Safety Consortium Virtual Conference Series. In a recent Q&A with Food Safety Tech, Mikael Bengtsson, industry & solution strategy director for food & beverage at Infor, explains yet another role that technology can play in helping companies maintain agility during changes that affect the supply chain such as the coronavirus pandemic.
Food Safety Tech: How can food suppliers mitigate the risks of foodborne illness outbreaks under the stress of the COVID-19 pandemic and with limited resources?
Mikael Bengtsson: Food safety must always be a top priority for any food and beverage company. The risks associated with contamination can have a severe impact for public health, brand and company reputation. Safety routines are therefore always of the highest priority. In today’s situation with COVID-19, the stress on safety is further increased. Now, it’s not only about keeping products safe but also keeping employees healthy. One progression and resource that all food suppliers must follow is the FDA [FSMA rules], which require suppliers to be diligent and document their compliance. Especially now, while suppliers are faced with limited resources and additional stress during the pandemic, they must rely on the basics—ensuring masks are worn in and out of the workplace, washing hands for at least 20 seconds prior to touching any food, and remaining six feet apart from co-workers. When it comes to a crisis like COVID, take solace in knowing suppliers can rely on the basics—even when conditions are strained.
This year we have seen many companies having to adapt and change quickly. Demand has shifted between products, ingredients have been in shortage and many employees have had to work from home. Some were better prepared than others in adapting to the new situation. Technology plays a big role when it comes to agility. Regarding food safety, there are many proactive measures to be taken. The industry leaders establish transparency in their supply chain both upstream and downstream, use big data analysis to identify inefficiencies, as well as couple IoT with asset management systems to foresee issues before they happen.
FST: How can technology help suppliers meet the growing consumer demand for transparency in an end-to-end supply chain and improve consumer trust?

Bengtsson: Communication with consumers is changing. It is not only about marketing products, but also to educate and interact with consumers. This requires a different approach. Of course, consumers are loyal to brands, but are also tempted to try something new when grocery shopping. After a new study is published or a new story is written, consumers are likely to shift their shopping preferences.
It is therefore important to build a closer connection with consumers. Companies who have full supply chain visibility, transparency and traceability have detailed stories to tell their consumers. One way they can build these stories is by including QR codes on their packages. The consumer can then easily scan the code and be brought to a website that shows more product details—e.g. who was the farmer, how were the animals cared for and what sustainability efforts were involved. These are all important aspects to build consumer trust. According to researchers at MIT Sloan School of Management, investing in supply chain visibility is the optimal way to gain consumer trust, and can lead to increased sales.
FST: What technologies should suppliers leverage to better collaborate with trading partners and ensure consistent food safety procedures?
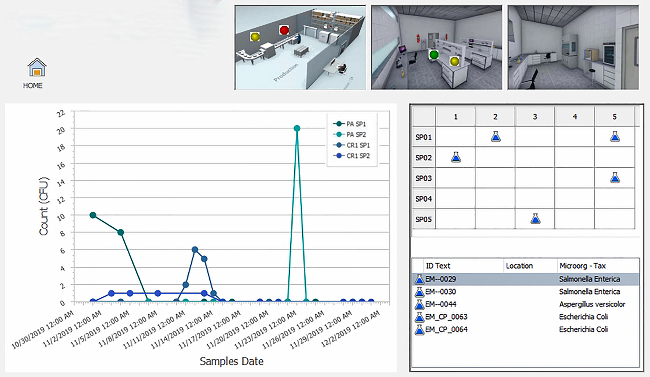
Bengtsson: When a food safety problem arises, batches, lots, and shipments need to be identified within minutes. Manufacturers must be able to trace all aspects of products throughout the entire supply chain—with complete visibility at the ingredient level—from farm to table, and everything in-between. An efficient and transparent food supply chain requires extensive collaboration and coordination between stakeholders. New technologies can extend both amount of collaboration possibilities and the impact of those collaborations. In order to maintain a transparent, efficient food supply chain, companies need to invest in modern cloud-based ERP and supply chain systems that incorporate the increased visibility of the Internet of Things (IoT) with data sharing, supplier and customer portals, and direct links between systems—all aimed at facilitating joint awareness and coordinated decision-making. Modern technologies that enable transparency will also have the added benefits of meeting consumer demand for product information, identifying and responding to food safety issues, reducing food waste, and supporting sustainability claims.