1/30/2025. Editor’s update of original article from 11/15/2024. Robert F. Kennedy Jr. battled his way through his second Senate confirmation hearing. According to the NY Times, Kennedy defended his views on vaccination during a raucous three-hour session on Thursday that featured shouting matches, angry accusations and a senator in tears, and also exposed the deep misgivings of a key Republican who could hold Mr. Kennedy’s future in his hands. The Senate hearings focused Mr. Kennedy’s views on vaccination did not touch on his food and agriculture ideas and plans.
Original article from 11/15/2024: According to the Wall Street Journal, President-elect Donald Trump is planning to nominate environmental lawyer Robert F. Kennedy Jr. to serve as Health and Human Services secretary. Kennedy’s nomination was not a surprise. Last month, Kennedy said Trump had promised him control of the department and its many subagencies, which include the Centers for Disease Control and Prevention (CDC), the Food and Drug Administration (FDA), the National Institutes of Health (NIH), the Centers for Medicare and Medicaid (CMS), and others. Trump himself pledged during the campaign to let Kennedy “go wild on health.”
To put things into context, the mission of the U.S. Department of Health and Human Services (HHS) according to it’s website is to enhance the health and well-being of all Americans, by providing for effective health and human services and by fostering sound, sustained advances in the sciences underlying medicine, public health, and social services. FDA is an agency within the Department of Health and Human Services and consists of nine Center-level organizations and thirteen Headquarter (HQ) Offices including the newly reorganized Human Foods Program headed up by Jim Jones.
Kennedy’s website, Make America Healthy Again states that Kennedy has spent nearly 40 years fighting corrupt corporations and government agencies. During his tenure at RiverKeeper, he successfully sued dozens of municipalities to force compliance with the Clean Water Act. He won cases against corporate giants, including a suit against General Electric for toxic runoff from its corporate jet hangar and a court order against ExxonMobil mandating they clean up tens of millions of gallons of spilled oil in Brooklyn, NY. As of Dec 2022, the Monsanto lawsuits to which Kennedy has devoted much of the past decade have yielded $11 Billion for farmers, migrant workers, day laborers, and families exposed to the pesticide RoundUp.
If approved, what can the food industry expect from Kennedy?
According to Make America Healthy Again, Kennedy sees Big Pharma and Big Agriculture having an undue influence on what Americans eat and how they manage their health over time.
Kennedy says the public health establishment is too focused on infectious diseases and wants to redirect resources toward issues he characterizes as the chronic disease epidemic, including obesity, diabetes, autism and mental illnesses. He blames them on corporations including food producers using harmful pesticides and additives. He traces America’s high levels of chronic disease to the widespread availability of highly processed, non-nutritious food, which he blames in part on a broken agriculture policy.
Some of his food and agriculture ideas plans share the same pseudoscience as Kennedy’s views on vaccines. Kennedy recently posted on social media that the FDA had “waged a war on public health” by “aggressive suppression” of Americans’ access to raw milk, despite raw milk’s risk of causing life-threatening diarrheal diseases and now, bird flu,
Kennedy has labeled Trump’s fast food diet as “poison” and wants to reduce the amount of ultra-processed food in the American food supply. In recent interviews, Kennedy has suggested clearing out “entire departments” at FDA, including the Center for Food Safety and Applied Nutrition.
However, if Kennedy wants to restrict the use of already-approved food additives, he needs more resources — not fewer: The process involves rigorous reviews of data, issuing public warnings, and actively monitoring the food supply. If Kennedy succeeded in closing the food safety office, that would reduce the number of people who could be dedicated to the job.
The European culture regarding food additives is generally the additive needs to be proven safe before allowed into the supply chain. Conversely, FDA’s position for years has been the additive is allowed to be used unless it has been proven harmful. Two opposite ways of regulating. Kennedy’s position on additives might move the U.S. to be more like the E.U.
Other actions could be taken by the Trump administration to reduce the amount of ultra-processed food in the American food supply, but many of them would be taken outside of HHS. The US Department of Agriculture (USDA) sets the guidelines that govern school lunch programs, which means much of what children eat is determined by that agency; Trump has not yet nominated a USDA commissioner. The USDA is also primarily responsible for overseeing farming, another industry Kennedy has heavily criticized throughout his public career and pledged to target if he were to take a role in the federal government. He would likely need to work with the USDA to follow through.
One Agency?
Kennedy is correct that food safety regulation in the US is currently a mess, says David Acheson, President of TAG. Meat, poultry, and egg plants are inspected daily under the auspices of the USDA, while every other kind of food production facility — including the farms whose produce is responsible for most of the food-borne illness in the US and the nation’s countless other industrial food manufacturers — are inspected by FDA inspectors at most once a year.
It would make far more sense to unify these functions under one agency and harmonize the frequency of food production facility inspections so none are falling through the cracks. That is the kind of organizational shake-up that could actually make a difference.
The concept of one agency is not new according to Frank Yiannas, former Deputy Commissioner for Food Policy and Response at the U.S. Food and Drug Administration. Yiannas, in his closing keynote presentation at last month’s Food Safety Consortium Conference said the idea has been proposed by the Obama and the previous Trump administration, however, it requires Congress to approve the combination of the two agencies.
It remains to be seen what impact Kennedy’s proposals will have on the food industry but they bear little resemblance to those of prior Republican administrations, which have typically favored cutting regulations, not increasing them.





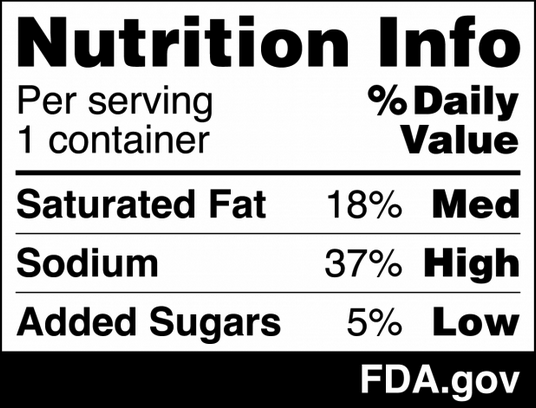




 Lead by Jim Jones, Deputy Commissioner for Human Foods at FDA, the reorganization establishes the HFP by realigning the functions of the Center for Food Safety and Applied Nutrition, the Office of Food Policy and Response, as well as key functions from the Office of Regulatory Affairs (ORA) under one program.
Lead by Jim Jones, Deputy Commissioner for Human Foods at FDA, the reorganization establishes the HFP by realigning the functions of the Center for Food Safety and Applied Nutrition, the Office of Food Policy and Response, as well as key functions from the Office of Regulatory Affairs (ORA) under one program.
