Patulin is a harmful mycotoxin produced by fungi typically found in damaged fruits, including apples, pears and grapes. Recently, researchers from Japan identified a new filamentous fungal strain that can degrade patulin by transforming it into less toxic substances, which may lead to new ways of controlling patulin toxicity in food supplies.
Patulin is produced by several types of fungi, and is toxic to humans, mammals, plants and microorganisms. Symptoms of exposure include nausea, lung congestion, ulcers, intestinal hemorrhages, and even more serious outcomes, such as DNA damage, immunosuppression, and increased cancer risk. Environments lacking proper hygienic measures during food production are more susceptible to patulin contamination as many of these fungi species tend to grow on damaged or decaying fruits, specifically apples, and can contaminate apple products, such as apple sauce, apple juice, jams and ciders.
In the recent study, the research team from Tokyo University of Science (TUS) in Japan, screened soil for microorganisms that can potentially help keep patulin toxicity in check. The team cultured microorganisms from 510 soil samples in a patulin-rich environment, looking for those that would thrive in presence of the toxin. Next, in a second screening experiment, they used high-performance liquid chromatography (HPLC) to determine the survivors that were most effective in degrading patulin into other less harmful chemical substances. They identified a filamentous fungal (mold) strain, Acremonium sp. or “TUS-MM1,” belonging to the genera Acremonium, that fit the bill.
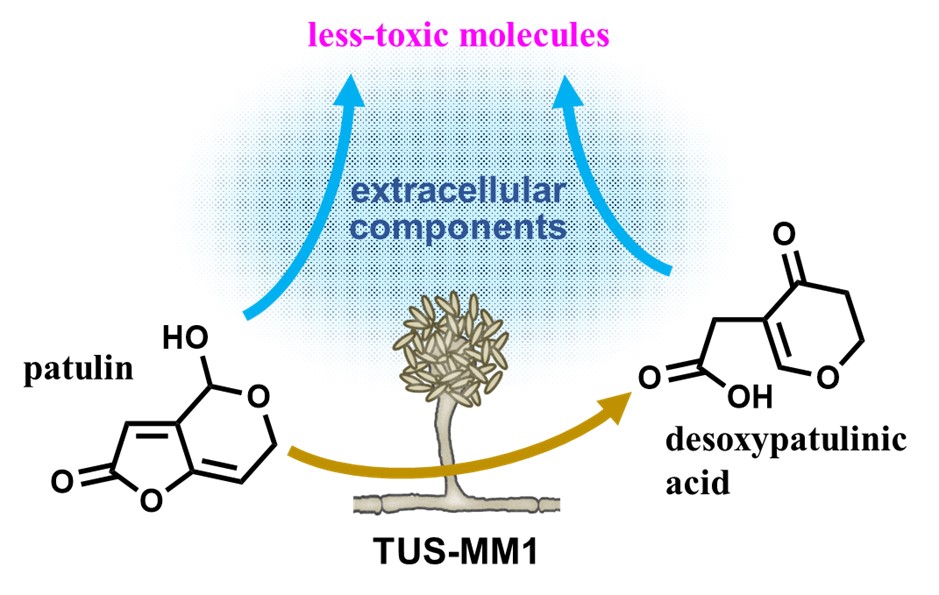
The team then performed various experiments to shed light on the mechanisms by which TUS-MM1 degraded patulin. This involved incubating the mold strain in a patulin-rich solution and focusing on the substances that gradually appeared both inside and outside its cells in response to patulin over time.
One important finding was that TUS-MM1 cells transformed any absorbed patulin into desoxypatulinic acid, a compound much less toxic than patulin, by adding hydrogen atoms to it. Moreover, the team found that some of the compounds secreted by TUS-MM1 cells can also transform patulin into other molecules. By mixing patulin with the extracellular secretions of TUS-MM1 cells and using HPLC, they observed various degradation products generated from patulin. Experiments on E. coli bacterium cells revealed that these products are significantly less toxic than patulin itself. Through further chemical analyses, the team showed that the main agent responsible for patulin transformation outside the cells was a thermally stable but highly reactive compound with a low molecular weight.
“Elucidating the pathways via which microorganisms can degrade patulin would be helpful not only for increasing our understanding of the underlying mechanisms in nature but also for facilitating the application of these organisms in biocontrol efforts,” said Dr. Toshiki Furuya, Associate Professor at the Faculty of Science and Technology of the Department of Applied Biological Science at TUS and co-author of the study.