Root cause analysis is a familiar term across various industries and a requirement at some level by most regulatory bodies. Unfortunately, the process of investigating a root cause may seem overly burdensome and consequently appropriate root cause tools are often abandoned. If the available root cause methods are viewed as tools within your toolbox, their value can be realized once the appropriate tool is selected. Consequently, with the right tool, an investigation is usually more efficient and successful.
A common definition of root cause is “The most basic reason a problem has occurred that once identified and eliminated will prevent reoccurrence of that problem.” In other words, the root cause is the “true” reason that contributed to the creation of a problem, defect or nonconformance. The critical first step in root cause analysis is identifying the problem. The problem statement should clearly state what is being investigated and be referenced by the team conducting the root cause analysis throughout the investigation to avoid getting off track. Note that I mentioned a team; often complex problems can not be properly investigated by an individual, and cross-functional input is required. Once the problem has been clearly stated and the investigation team formed, it is time to look in the root cause toolbox for the best available tool.
One of the most used root cause tools is the “Five Whys.” It is useful when you want to quickly get to the root cause of a simple problem. It can also be used prior to the “Fishbone” or in conjunction with other tools (such as “Is/Is Not”) and is typically best for lower risk problems. The Five Whys tool was originally developed by Sakichi Toyoda and used within Toyota Motor Corp during evolution of their manufacturing methods. It is a problem-solving technique that is the basis of a scientific approach in which by repeating “why” five times the nature of the problem as well as its solution becomes clear. The objective is to get the investigator to avoid assumptions and logic traps and trace the chain of causality from the problem through layers of questioning, logic, and answers to the root cause. Always ask yourself if another “why” can be asked before you conclude the investigation activity and test the conclusion by using “therefore” statements in reverse. If you can not ask another why, you can finalize the Five Why investigation activity and summarize the root cause.
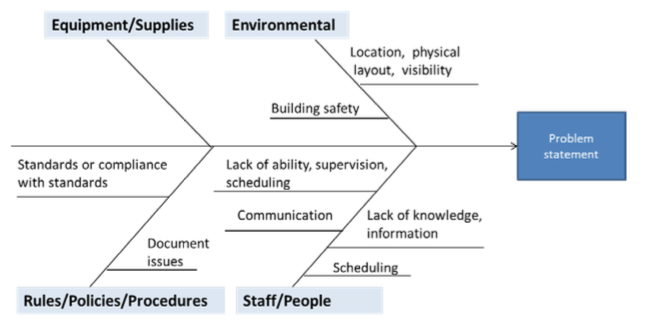
Another root cause tool in the toolbox is the “Fishbone Diagram,” which can prove useful when tackling more complex problems. To use a fishbone, first document your problem statement and then draw a fish skeleton (or use a fishbone form) with spines labeled Manpower, Method, Machine, Material and Environment (or their equivalent terms). Use evidence-based techniques to identify possible causes (brainstorming, employee interviews, watching video, drawing process maps, reviewing documents, etc.) and add the facts to the correct category. Eliminate causes that don’t apply; analyze the diagram and designate the causes that do most likely apply. Use data and critical thinking to determine the root cause. It is recommended to use team input for the final selection.
When you need to focus a brainstorming session and narrow down solutions, you may want to use the “Is / Is Not” tool. The Is / Is Not tool is a comparative analysis method that can be used to determine root cause or focus an investigation and is fairly simple to use. To begin, draw a diagram with a column for “Is” and a column for “Is Not.” Next, ask “Is” questions. Gather relevant data on the problem and what actually happened and add it to the “Is” column (what is wrong, where is the problem occurring, when did it go wrong, how often does it go wrong, etc.). Then, ask “Is not” questions. Make comparisons to the “Is” column to set boundaries (what is not wrong, what else could have occurred but didn’t, what lines has it not occurred on, when could it have occurred in the past, etc.) and list these in the “Is Not” column. Look at the two columns to obtain clues for the root cause and then make a conclusion or set an investigative strategy to determine root cause. The Is / Is Not tool can be a practical way to refocus an investigation and is often used in conjunction with other tools.
Unfortunately, a common pitfall with all these tools is determining a root cause of “human error.” Do not immediately assume “human error” is the root cause, though it may seem like an easy conclusion. A thorough root cause will analyze “why” the human made the error and address that root cause. An organization with numerous “human error” root causes is a red flag to investigators and an indication that the true cause of the issue is not being properly investigated or addressed. As Dr. W. Edwards Deming wrote in his book “The New Economics”1: “People can’t perform better than the system allows.” Consider if your processes and systems are setting employees up for success or failure and address accordingly.
To summarize, there are many root cause tools available when conducting an investigation, but just like with an actual toolbox, picking the right tool for the job is critical. “Five Whys” is best for lower risk or more simple problems than can be quickly solved. A “Fishbone” diagram is useful for more complex problems, especially when there is a lot of data to organize and evaluate. “Is / Is Not” is often an auxiliary tool than can help further define a complex problem when other tools are unsuccessful or to identify boundaries of an investigation. Additionally, there are other root cause tools available, such as Failure Mode and Effects Analysis (FMEA), Control Charts, and Affinity Diagrams than can be used as a supplement to the above methods or alone. Regardless of the tool used, the key to investigational success and prevention of recurring errors is a well documented and scientifically sound root cause investigation.
References
- “The New Economics” (3rd edition, 2018): Book by the late W. Edwards Deming