

On March 19, 2024, the USDA implemented revised regulations on organic foods. The overall purpose of the revisions is to strengthen enforcement of the regulations, with a focus on greater accountability for organic food fraud.

On March 19, 2024, the USDA implemented revised regulations on organic foods. The overall purpose of the revisions is to strengthen enforcement of the regulations, with a focus on greater accountability for organic food fraud.
The FDA’s Food Safety Modernization Act (FSMA) is designed to enhance food safety by establishing and regulating traceability requirements. The act mandates that all parties in the supply chain share critical information to address potential food safety issues. This article discusses FSMA requirements for comprehensive recordkeeping, including defining Critical Tracking Events (CTEs) and Key Data Elements (KDEs). It highlights the crucial role of Electronic Data Interchange (EDI) in meeting compliance and optimizing food traceability. EDI automates data exchange, improves visibility, simplifies compliance, and enables rapid outbreak response. By investing in EDI technology, organizations can establish a strong foundation for complying with FSMA regulations and ensuring food safety.

When food and beverage manufacturers aim to increase total throughput, they often face facility size constraints. Manufacturers can either expand their current facility or move operations to a larger building.

Maintaining the safety of the food we consume is complex and multi-faceted. Inspections and audits have become the backbone of our food industry’s quality control processes in preventing foodborne illnesses and ensuring the stringent regulatory measures are met. This article details the intricacies of the importance of inspections and audits, highlighting the procedure involved to reduce the consistency of recalls.

Following the October 2023 recall of cinnamon apple puree and applesauce products due to elevated lead levels linked to the cinnamon in those products and the concern for lead toxicity in children, the FDA initiated a targeted survey of ground cinnamon products from discount retail stores and analyzed the samples for lead and chromium.

The proposal would establish final product standards to prevent raw chicken carcasses, chicken parts, ground chicken, and ground turkey products that contain any type of Salmonella at or above 10 colony forming units (CFU) per gram/ml and any detectable level of at least one of the Salmonella serotypes of public health significance from entering commerce.
Although there is no definitive method to certify compliance with food safety culture, numerous approaches exist to demonstrate that a facility and its employees are thoroughly engaged in proper food safety practices.
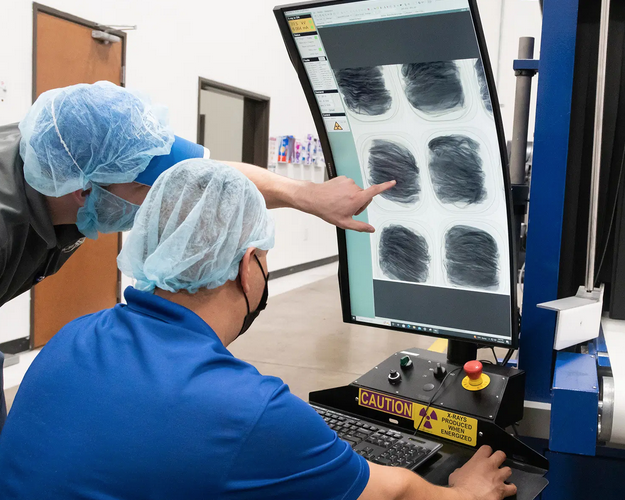
Foreign material contamination in food production is a widespread issue that can occur at any stage of the supply chain, demanding rigorous detection and traceability measures. Contaminants can originate from all phases of manufacturing—from raw materials to processing and production—and can pose significant safety risks and operational challenges. Utilizing advanced technologies and third-party inspection can help mitigate these risks, ensuring product safety, protecting brand reputation and maintaining regulatory compliance.

Purchasing gloves with FDA “food compliance” alone may not be a sound food safety strategy. In this article, the author provides five key pieces of advice on glove selection and implementation to ensure they remain the foundation of food safety.

A gap assessment can help determine what requirements existing traceability programs already meet and identify where improvements are needed to comply with the final Food Traceability Rule by the January 2026 deadline.