According to a report by Kroll and The Economist Intelligence Unit, 17% of companies experienced some type of vendor, supplier or procurement fraud in 2015. While fraud is one of the more extreme examples of supplier management complications, the manufacturer-supplier relationship is notoriously fickle and can result in serious issues if attention and care is not reciprocal from the beginning.
With great communication and even better processes in place, your suppliers have the potential to become strategic partners for your brand, helping drive your values across the supply chain while also helping you achieve overarching business goals.
Do Your Homework
In order to foster positive supplier relations, it is important to consider all available options and carefully assess them before engaging. In the research phase, it is critical to get as many references as possible to ensure you align with a potential supplier when it comes to safety practices and brand values. Looking at a supplier’s history is an effective way to gauge how your partnership will pan out and catch any red flags before they become a bigger problem for the brand, whether that be poor communication habits, dishonesty about products or inconsistent record keeping.
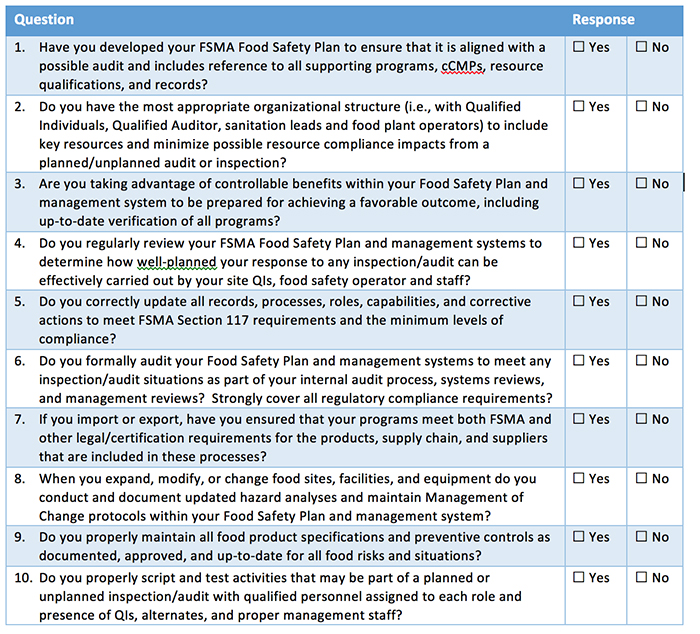
FSMA deadlines for compliance with the Foreign Supplier Verification Program (FSVP) are right around the corner. With the changing regulatory landscape, thoroughly investigating potential suppliers is crucial, especially if they are outside of the United States, as the stakes are much higher. Under the FSVP, importers are essentially “guilty” until proven “innocent”—a sharp contrast from how foreign suppliers were previously handled by the FDA. The standards for imported food are stricter than ever, as are the consequences for companies that are found working with foreign suppliers without verification. With the FSVP, the FDA can halt all importations completely as long as they have reason to believe the supplier is not compliant with the program.
Communication Is Key
At the cornerstone of any good relationship is communication; the same goes for relationships within the food industry.
Once a supplier has been thoroughly vetted and is officially on the team, the key to maintaining a successful relationship is transparency. Without full transparency with suppliers, you can’t offer consumers reliable information about their food. At the same time, manufacturers need to be straightforward with their needs to ensure suppliers are able to uphold their expectations. By thoroughly communicating plans and expectations, you and your suppliers can effectively work together to achieve future goals.
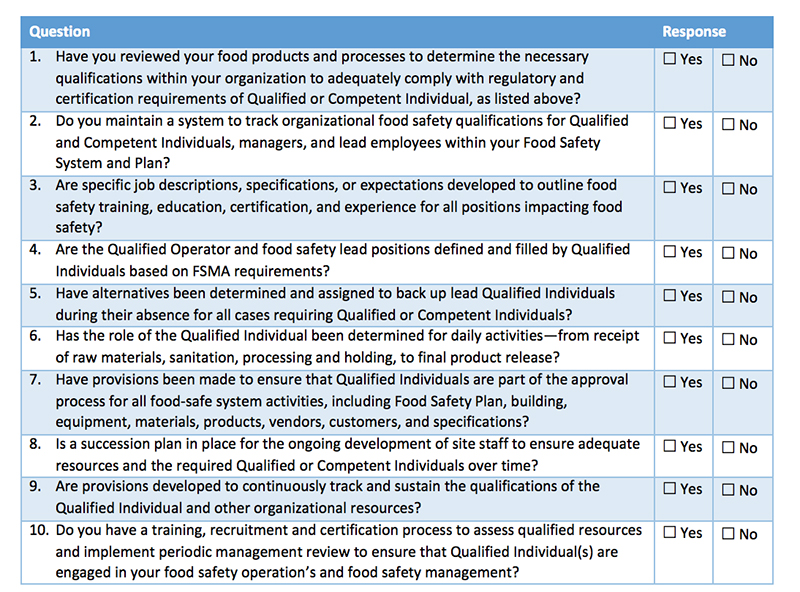
At the start of a working relationship with a supplier, it is important to comprehensively onboard and train them in your plans and processes to avoid a lack of understanding down the line. By setting up an all-encompassing onboarding system, inclusive of checklists and background documents on procedures and standards, you can help ease growing pains and empower your new food supplier to become a trusted partner. For instance, if you use a specific supply chain technology, your suppliers should know ahead of time so they can receive adequate training on the solution. This will help streamline communication and minimize any bumps in the road.
Regular Check-Ups
While safety and contamination issues are undesirable, they are inevitable. When faced with an outbreak or contaminant in your supply chain, suppliers become your most crucial resource. A poorly handled recall can wreak havoc on a food manufacturer, with the potential to ruin a trusted brand. Having the correct protocols in place with suppliers to ensure proper procedures are followed quickly and efficiently is critical. In order to make sure suppliers are complying with standards, keeping complete records and maintaining proper safety practices, it is essential to perform regular supplier audits.
With the addition of new technologies in the last few years, monitoring supplier performance and implementing corrective actions has never been easier. There are companies that offer supplier management and food safety management software to enable manufacturers 24/7 end-to-end visibility into their food supply chain and suppliers’ practices, while simplifying communication. Supplier management software offers a single platform that allows a brand to safeguard important supplier documentation, submit proper records to regulators when audited, streamline supplier audits and compliance records, and communicate corrective actions.
Overall, supplier management software with end-to-end supply chain visibility is a great way to keep up with suppliers and rest assured that your company’s food safety guidelines are being followed at all times.
Keeping Consumers Safe and Happy
With the current state of food safety, keeping suppliers in check is absolutely crucial for brands. As the FDA is increasing regulations with the adoption of FSMA, manufacturers must be able to trust their suppliers to uphold these new standards. If there are any slip-ups, your brand is held accountable. At the same time, with the increasing number of high-profile recalls and foodborne illness reports, consumers are on high alert, and winning their trust is harder than ever; today’s conscious consumer expects total transparency from their food brands, something only achieved through a strong supplier management program.
Fortunately, given advancements in technology, manufacturers can now foster more proactive relationships, assess supplier performance and achieve mutual goals across the chain smoothly.
While good supplier management requires time and resources, it is worth the investment. Putting in the effort to foster strategic partnerships with suppliers is key to mitigating safety and contamination issues, meeting the FDA’s regulations, as well as keeping consumers safe and happy.