FSMA will add more responsibility to a laboratory’s plate, stressing the need to maximize research and develop an integrated approach to prioritizing risks. Under its general requirements, research and regulatory labs will be expected to examine performance standards, cooperate with federal partners within HHS and the Department of Homeland Security, and build a domestic capacity that encompasses federal, state and international partners.
Partnerships between research and regulatory labs should strive to bridge information gaps with the goal of harmonizing standards, integrating lab networks, and expanding surveillance programs. During the Food Labs Conference in March, Palmer A. Orlandi, PhD, CAPT, U.S. Public Health Service Sr. Science Advisor in the Office of Foods and Veterinary Medicine at FDA, discussed how partnerships in the era of FSMA are crucial to facilitate innovation. “We’re not necessarily looking for someone to take our responsibilities, but we’re looking for someone to walk with us to do this,” said Orlandi.
For research and regulatory analytical capabilities to move forward, several needs and goals must be addressed:
Needs
• Burden sharing
• Expansion of the scope of testing programs (and the methods to support them)
• Development of sampling strategies
• Risk-informed prioritization strategy
Goals
• Capacity building
• Methods that are rapid, sensitive, specific, easy to use, robust and portable
• Ability to test at the source
• Database of information that shows susceptibility for contamination and root cause, while also providing solutions for prevention
• Targeted and statistically significant surveillance, with the ability for sharing
Examples of capacity-building partnerships include the Food Emergency Response Network (FERN), which is run by FDA and USDA. FERN is comprised of more than 170 state and federal labs, and has gone beyond its roots in emergency capacity, expanding into a food safety network that also participates in large-scale surveillance. The Integrated Food Safety System incorporates a Lab Task Group with seven subcommittees to develop standards in areas that include accreditation, methods, regulatory requirements, reporting, and sampling. International partnerships are currently being forged in Mexico and Canada.
| View excerpt from Palmer Orlandi’s presentation about Partnerships & Innovation at the Food Labs Conference |
What’s Next: Innovation, Technology and the Possibilities
Portable technology: A user-friendly, handheld rapid-screening instrument that requires minimal sample prep and is cost effective. Think Tricorder. Will it be possible to wave an instrument over a head of lettuce and detect bacterial contamination? What about detecting a spectrum of approved or unapproved pesticides or active pharmaceutical ingredients?
“This is where we would like to go,” said Orlandi. “Is it pie in the sky? Absolutely. But if you don’t ask the big questions, if you only take the incremental steps, you’re only going to get so far.”
Orlandi pointed to X-ray fluorescence, which takes less than two minutes to perform sample analysis and ion mobility spectrometry, which can detect a small range of selected compounds in just 30 seconds, as technologies that have future potential.
FDA has a goal of bringing such innovative technologies to bear through its Broad Agency Announcements, a program that provides funding from $200,000 to $50 million to harness new technologies.
Orlandi also cited the Whole Genome Sequencing (WSGS) Collaborative as the next big technology. The GenomeTrakr is a federal and state network of 24 labs that collect and share genomic data from foodborne pathogens. This enormous data flow provides the ability to sequence and transmit and store data, involving domestic and international partners. One application example includes identifying antimicrobial resistance markers.
As FSMA increases industry requirements, “partnerships are going to spawn our capabilities to harmonize standards that will involve, then leads to mutual reliance,” said Orlandi. “We rely our partners’ data and their processes. This will lead to greater capabilities for surveillance and data sharing. All of these combined will lead to a greater food safety network.”
Related Content: Five Questions with Palmer Orlandi



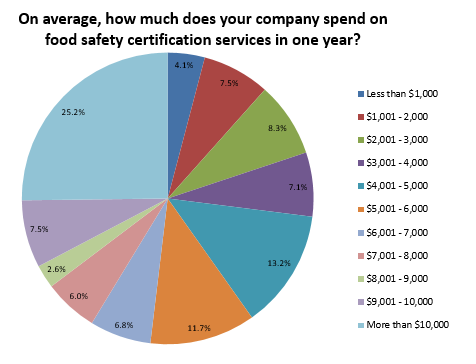


 Ultimately, the following factors influenced food companies’ decision to go through food safety certification:
Ultimately, the following factors influenced food companies’ decision to go through food safety certification:

 Lack of trend analysis of environmental data;
Lack of trend analysis of environmental data;





