In America’s food supply chain, food is sourced globally. Since ingredients often come from multiple countries, inspection and quality control is challenging, as regulations, policies and processes differ in each country. Product management begins with the suppliers, from the fields where the foods are grown, to the pesticides and fertilizers used, to harvesting, washing, shipping, storing, and processing (manufacturers), and finally, to packaging and delivery to consumers.
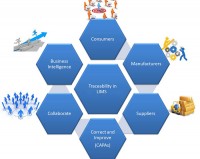
Figure 1 shows each step of the product management process can introduce contamination due to unsafe practices or other risks. As such, test data and traceability must begin in the field and end when the final product is delivered to the consumer. The Laboratory Information Management System (LIMS) captures all information to ensure that quality data is effectively managed, communicated, and easily and quickly accessible in the event of a contamination issue. The LIMS allows producers to provide authorities with the required sampling and testing documentation to prove compliance.
U.S. consumers expect their food products to be affordable, consistent, safe and unadulterated. Consumers have seen numerous food recalls in the news, and it has shaken their confidence. The CDC estimates that about one in six Americans (or 48 million people) get sick, 128,000 are hospitalized, and approximately 3,000 die of foodborne diseases each year. Global food directives for international food initiatives include CODEX, ISO (International Standards Organization), and the Global Food Safety Initiative (GFSI).
The U.S. Government has implemented various food safety programs, from Hazard Analysis & Critical Control Points (HACCP) to FSMA in order to identify and correct potential contamination in the food supply. In fact, one of the primary focuses of FSMA is preventive action based on risk assessment.
The food landscape has changed significantly, especially over the past decade, as consumers demand year-round fresh fruits, vegetables and juices, along with more exotic foods. The fact that U.S. food is globally sourced has resulted in numerous challenges in quality assurance, shipping, traceability, labeling, storage, blending, testing, and reporting.
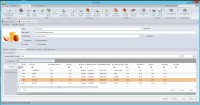
For example, upon reading the labeling on an apple juice can, it is not uncommon to learn the juice has been possibly sourced from numerous countries including the United States, China, Brazil, Argentina, Chile and many other countries from the European Union. Oftentimes, labels state that ingredients may come from some of the countries listed, but it does not specify what percentage comes from each country or exactly from which country the product was sourced. Figure 2 shows how LIMS can track and manage this information in a relational SQL Server LIMS database.
A similar scenario is true for tracking hamburger meat: The meat that was used to make burgers can come from multiple ranches and hundreds of cows. Many consumers don’t understand why their food/beverage is blended in large ton batches, and producers want to reach the required final product specifications, while offering a consistent product and experience to the consumer. Blending has become commonplace in the food industry, and it makes traceability much more challenging. The same is true in blending different meats, for example regulators have found pork in products marked 100% beef, this has led to the use of molecular tests to determine if meat has been adulterated.
FSMA and Traceability
FSMA focuses on a preventive approach rather than reaction and response to foodborne outbreaks. A central focus is on traceability, involving a complete understanding of the complex food chain and conducting testing at the key control points that can introduce contamination. It is important to understand the source of all the raw ingredients that make up a final product as well as the details of where they are sourced, the CoA (Certificate of Analysis) report, other test results, and all associated documentation. These elements are especially important, because each region of the world has different approved testing methods and is challenged with different potential contaminants and processes. As a result, food manufacturers must manage a significant amount of information on all raw materials that they receive, along with the associated paperwork, which includes the CoA, confirmatory test data, and all plant, production and final product test data.
| Case example. As operations scale, so does the testing. In order to manage all the testing, most laboratories turn to LIMS and laboratory automation to manage high throughput screening. A client that was performing nearly 1,000 Listeria tests per day was using an automated microbiological screening platform to complete this testing. They were struggling to hire more resources to manage and run the instrument, as the time was short and the increased sample volume was imminent. The goal was to automate testing from the nine plants that were submitting samples to the main laboratory, such that the entire process could be automated from the laboratory knowing how many samples were coming from each plant and from deploying pre-configured worklists to upload to the instruments. The instruments would then run the samples and send the result back into the LIMS. This integration alone saved more than six hours per day. In addition, the electronic data transfer was fast and error-free, and since the data was imported into the LIMS, any positives were automatically flagged in real time. This approach allows immediate action. |
In addition, all data from shelf life studies and additional testing on the food product (i.e., pesticide testing, environmental testing for Listeria sp., mold, yeast, etc., formulations, and blending) can be managed in the LIMS, one centralized database.
How LIMS Supports FSMA
Over the years some manufacturers have relied on less-robust tools to manage and maintain testing data, from multiple Excel spreadsheets to paper log books. Challenges with using these tools include data corruptions, data loss, typographical errors, and accidental or malicious data changes. These systems are often costly, especially from a resource standpoint (i.e., data errors, hours spent interacting with the data for calculations, tracking samples, and manual report creation alone). In addition, creating reports for regulating authorities can be time-consuming and because there is no control over changes to the Excel sheets or logbooks, there is typically no audit trail, and because the data is not in the database, querying the data can be very difficult.
A quality LIMS will ensure that the organization is bullet-proof when it comes time for regulatory audits. It also provides a complete and secure solution to manage, track and monitor batches of product from farm to table. LIMS not only helps clients manage their regulatory compliance goals, but it also facilitates communication across the organization and provides laboratory intelligence that gives buyers insight into the best suppliers to purchase from, based on final product specification, consistency and pricing. Managers can also better understand when it is time to outsource testing based on workload data, allowing them to maximize their resources and profitably through more efficient operations. The system also accelerates communication: As soon as testing is completed, reports can be automatically emailed and alerts sent to cell phones, if any issues arise.
When dealing with perishable products, time is of the essence, LIMS save time. Table 1 lists just a few of major benefits of the LIMS in FSMA regulatory compliance.
| Process/Requirement | Advantage |
| Sample tracking and management | Integrated barcode support (both 1D and 2D), manage all batch data, tests, from raw materials, in process testing to final packaged product testing |
| 21 CFR Part 11 | Compliance with electronic signature requirements |
| CoA | Easily, automatically generate the CoA report once testing is completed, validated and approved |
| Specification Management | Manage final product, supplier and customer specifications and pricing |
| Document Management | Link all paperwork to Work Order for ready access and retrieval |
| Full Chain of Custody | Automatically generated and linked to the order |
| Records data and all paperwork associated with product | All paperwork that arrived with the raw ingredients, CoA, and shipping documentation or additional test data |
| Records all test results | Automatic data import from instruments as well as hand entered data |
| Shelf-life Studies | Setup, manage and track all aspects of shelf life studies |
| Formulations and Blending | Manage and track as components and specifications for final product blends, and leverage predictive tools for optimal purchase options from suppliers |
| Audit Trail | Track actions in the system and generate a report of all audits made to any result data |
| CAPAs (Corrective and Preventative Actions) | Track and manage open CAPAs in the LIMS, and tie to testing results for easy management to increase customer satisfaction |
| Traceability back to the source (farm, country) and forward to the store that it was shipped to, with key data (lot number, ship date, etc.) | Users can view all components and associated test results, along with any notes on the final product, back to the supplier and forward to locations that offer the product to the consumer |
| Employee Training | Manage employee training records and view Standard Operating Procedures online to ensure access to work instruction and provide evidence for audits |
| Instrument Management | Manage all quality control data on the instruments used in the testing, as well as documented calibration data, maintenance, any repairs, or any issues. Users can link the PDF manual in the LIMS |
| Enterprise integration (ERP, SAP, SCADA, MES, SAS JMP) | Data sharing allows users with permissions access to data when they need it, so that they can quickly view and monitor information they need to perform their job. Users can also view data with integrated statistical tools to view trends that may not be readily evident |
| Table I | |
A LIMS is a critical tool to the success of food companies. It organizes and securely manages all aspects of food testing, facilitates regulatory compliance, enhances communication within the organization, and maximizes productivity. Many food producers are concerned about protecting their brand and providing a high quality, consistent, and safe product to consumers while operating efficiently and at a profit. An LIMS allows them to meet these goals.
























