With nearly 40 years of experience in the food retail and manufacturing sectors, Swoffer has held a number of senior executive positions, including Head of Technical Services at British Retail Consortium, where he was instrumental in developing the BRC series of standards and the Safe and Local Supplier Approval scheme (SALSA). He has also worked at Nestlé UK and Safeway UK in several technical roles, and most recently served as a consultant to a number of global organisations.
“Kevin, Stephen and Grace have a great deal of technical experience in the industry,” said David Richardson, Vice President of NSF International’s Global Food Division’s EMEA region. “I have no doubt our clients will benefit from the changes we have made to our senior management team, adding a unique level of expertise and experience to the services NSF International provides.”
“I have worked with NSF International over many years and welcomed their professional input into standards development and certification services. NSF International has also developed a number of unique solutions to food safety issues. I have long respected their leadership and approach within a very demanding industry,” said Swoffer. “I’m confident we will continue to bring innovative technical services to clients and further enhance our consulting proposition.”
In his role, working as a member of the NSF Technical Services and Consulting Leadership Group, Swoffer will be responsible for developing, implementing and continuously improving services consistent with corporate strategy and meeting the needs of NSF clients. He will provide technical support, advice and guidance to members of NSF teams in the EMEA region.
Swoffer has been involved with the development of food safety standards since 1993. He has authored a number of industry publications including editing the UK Industry Guide to Good Hygiene Practice: Retail Guide, 2nd Edition and writing BRC Product Recall Guidelines. He was appointed as an expert on food quality and safety private standards for UNIDO (United Nations Industrial Development Organization) in 2009 and as the Chairman of the Global Food Safety Initiative (GFSI) Technical Committee in December 2007. He was one of the founding members of the GFSI in 1999 and has been actively involved with GFSI development in recent years. He holds a degree in food science and is a Fellow of the Institute of Food Science and Technology.
Stephen Cox, formerly NSF Agriculture International Development Director, moves to Global Managing Director, NSF Agriculture. In this new role he is developing new services for traditional and emerging agricultural markets in addition to further developing the business’ role. In his earlier role as Business Development & Quality Director for NSF Certification, Cox and his team managed many of the technical and integrity issues for a variety of pre- and post-farm gate assurance standards in addition to liaising with the global network with specific responsibilities for the U.S., Spain, Italy and South Africa.
Grace O’Dwyer has been promoted to the role of Director of Operations EMEA from her previous position as Director of Operations for NSF Agriculture. In her new role, O’Dwyer will focus on developing the European infrastructure of offices, technical expertise and administration support across Europe, the Middle East and Africa. This includes putting in place innovative IT platforms and systems and strengthening operating processes and systems to provide best-in-class customer service support. With a background of technical services provision and business development in the agri-industry, O’Dwyer has significant experience developing customer-led solutions and operating practices in international supply networks.
To learn more about the NSF Global Food Division, visit the NSF Food Safety website.

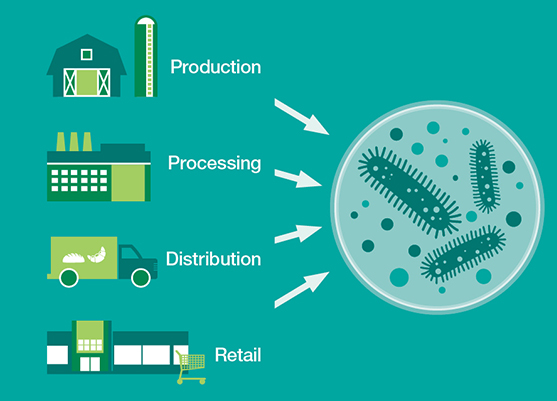
 One of the goals of the consortium is to see if there are any actions that food producers can take in respect to microbiomes that can reduce risk and make production safer. And Mars, with more than 130 factories worldwide, can help map the flow of microorganisms into and through the supply chain on a global level. An informatics infrastructure developed in the IBM Accelerated Discovery Lab, a data and analytics hub for IBM researchers and their clients and partners, will help the team parse and aggregate terabytes of genomic data from Mars and apply decades of refined analytics to uncover new insights. Adding relevant weather, transport and other contextual data could help define a targeted breakout, marking on the index a warning for food producers and distributors at the outset of a processing cycle.
One of the goals of the consortium is to see if there are any actions that food producers can take in respect to microbiomes that can reduce risk and make production safer. And Mars, with more than 130 factories worldwide, can help map the flow of microorganisms into and through the supply chain on a global level. An informatics infrastructure developed in the IBM Accelerated Discovery Lab, a data and analytics hub for IBM researchers and their clients and partners, will help the team parse and aggregate terabytes of genomic data from Mars and apply decades of refined analytics to uncover new insights. Adding relevant weather, transport and other contextual data could help define a targeted breakout, marking on the index a warning for food producers and distributors at the outset of a processing cycle.
