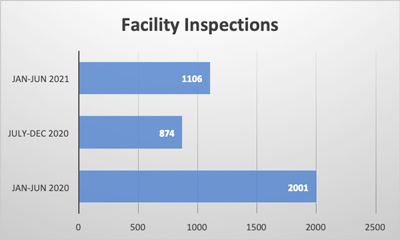
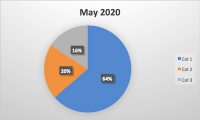
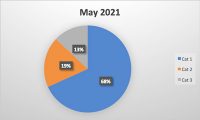
Packaging is an essential component of our modern, global food supply. While it helps us preserve and protect food and creates instant brand recognition for consumers, packaging also inserts an additional level of necessary risk mitigation into the manufacturing process. Liability stemming from packaging is a serious concern for food manufacturers of all sizes, with millions of dollars and brand-damaging lawsuits on the line. Processed foods need packaging to arrive in the hands of consumers, and processed foods are necessary to feed the world’s population. According to a survey conducted by the United States National Library of Medicine, “60% of calories consumed were from ultra-processed foods.”1 This shows the prevalence of processed foods and the significant impact packaging, a ubiquitous component of processed foods, plays in the food industry.1 However, if not handled properly, food packaging can be a significant liability.
Liability from packaging commonly presents in two ways: First, as foreign material contamination. Broken, damaged or defective packaging material can end up in food products, which increases the risk of a consumer attempting to consume contaminated goods. Second, packaging can be a hurdle to effective remediation of foreign material contamination, as goods can often not be efficiently or effectively inspected back through in-plant critical control points. The resulting disposal of product can contribute to food and environmental waste, as well as lost profits.
The harsh truth is that if manufacturers lack processes to identify, prevent or mitigate foreign material contamination when it occurs in packaged food, packaging can be a significant liability at every stage from the manufacturing facility to the store shelf. Following strict food safety plans can save countless hours, resources and dollars in the long run.2
Foreign Material Contamination: Where It Comes From
Foreign material contamination comes from multiple sources in the production cycle. It can come from raw materials, like animal bones ending up in boneless meat products. It can happen during the production process when a screw or seal detaches from a machine and gets mixed into a pie. It can be biological, like an insect ending up in a bag of chips. Or it can come from packaging: A shard of glass winding up in a jar of salsa or a plastic wrapper finding itself in a muffin. All of these foreign material contaminants are risks and dangers for which manufacturers can be held liable.
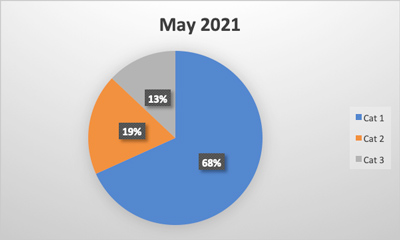
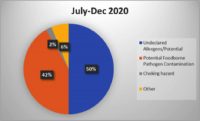
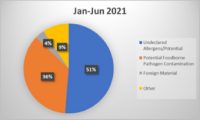
Packaging-related contamination is a high priority for manufacturers. Foreign material contamination due to packaging occurs when contaminants like metal, plastic, styrofoam and other objects end up where they do not belong, disrupting the integrity and quality of the product. Packaging materials can break down into tiny pieces that inline inspection machines may not be able to identify. Inline machines are calibrated for a certain size and certain types of foreign material contamination, which may not include packaging materials. Additionally, inline machines are often used at critical points during the manufacturing process but are not commonly used to inspect completed packaged products.
Break It Down: Liabilities Within Food Packaging
The party most affected by missed foreign material contamination is the consumer. Current consumer trends point to greater ingredient awareness, education and research into the companies from which consumers purchase products. The consumer mindset of environmentally friendly products and socially responsible purchases are impacting the food industry directly. Consumers seek transparency from brands about the products they’re ingesting. When a consumer discovers foreign material contamination inside a product, it creates frustration and eliminates trust. In addition to negatively impacted brand reputation, a foreign object from packaging can be incredibly costly. Recalls, especially those that require a local or national public warning, are detrimental to a brand’s reputation.3 Consumer trust in a brand is priceless and can take years to repair when broken.
In the age of social media, consumer reports of foreign material contamination can spread like wildfire across multiple platforms, reaching countless consumers across the world. One tweet about a contaminated product can go viral, costing corporations their reputations or worse–– lawsuits. An accidental miss somewhere along the production line can result in public outrage and cost the manufacturer millions of dollars in wasted product and crisis management. Suppose a consumer accidentally consumes a foreign contaminant from product packaging which results in injury. In that case, the manufacturer could be held liable for the medical bills and even long-term care if the injury is debilitating. These court cases can be highly costly monetarily and in terms of public perception.
In addition to legal liability from consumers, the loss of product for foreign material contamination is typically very costly when labor, storage, time, materials and lost revenue are taken into account. A producer who makes the moral and ethical decision to dispose of product that may be contaminated loses money doing so. They also risk harming their reputation with consumers by contributing to the problem of food waste.
In the 21st century, shoppers are increasingly looking “beyond the label,” and are concerned with the impact their purchase behaviors have on the environment.4 Consumers in younger demographics are significantly more likely to have a purchase decision influenced by a company’s impact on and concern for the environment. Packaging is a major concern for food manufacturers as they seek to lessen their environmental impact to meet market demands. This impacts foreign material contamination in two important ways. First, as packaging materials move towards the use of biodegradable materials, the capability of technology to detect the difference between packaging and food material must increase. Second, environmentally-friendly packaging is still relatively new compared to traditional materials, and the risks of foreign material contamination associated with these materials are still relatively unknown.
Manufacturers are in a difficult position when dealing with the liabilities stemming from packaging as a foreign material contaminant. Compounding this issue is the role packaging plays in preventing manufacturers from using in-house processes or inline equipment to inspect product back through Critical Control Points. Inline mechanisms for identifying foreign material contamination are not designed to inspect completed, packaged product. If producers dispose of and rework product, they risk harm to sustainability-focused brands, production quotas and bottom lines. If they attempt to identify the contamination themselves, they lose valuable production time and potentially lose perishable product to spoilage. With nearly every solution, another liability arises.
Packaging Contaminants: Prevention, Response and Liability
The FDA-required Hazard Analysis and Critical Control Points (HACCP) plan has seven principles to ensure manufacturers meet food safety goals from production to consumption. Physical, chemical and visual tests are involved to ensure foreign contaminants do not exist in products produced in the manufacturing facilities.5 The more detailed processes are in place, the more protected companies are from recalls and reducing the chance of a lawsuit where the manufacturer is liable. Implementing different programs and processes to prevent and diminish food waste ultimately positively impacts the manufacturer’s bottom line. The Business Case conducted a study called “Reducing Food Loss, and Waste” that found “99% of companies earned a positive investment when they implemented programs to reduce food waste”.6
Many companies engage third-party food inspection partners as an extra measure to ensure that their product is free from foreign material contamination. By electing to utilize third-party inspection services, manufacturers hit a trifecta: They can typically salvage the majority of on-hold product, reduce food waste, and with the right partner, get the data they need to have traceability of foreign material contamination issues at their plant.
Manufacturers should pursue third-party inspection partners that meet a high standard of excellence. The best third-party inspection partners use cutting-edge technology to inspect products in higher detail than inline machines. Their machines should be capable of identifying foreign material contaminants of all types and have a high capacity to turn around large volumes of product efficiently. Their machines should be capable of overcoming the obstacle of packaging as an impediment to inspection using machines with a larger aperture than typical inline tools. Lastly, third-party inspection adds significant value if it has the ability to find and retrieve foreign material contamination so manufacturers can effectively use the resulting data to identify and remediate problems within the plant. An inspection service that does not meet these criteria is not an inspection service, but merely a method for outsourcing the same practices that a manufacturer would conduct in-house.
Liability Questions Answered
So, when are companies liable for packaging issues? Unfortunately, the answer isn’t always black and white. FSMA is in place to help prevent foodborne illness, requiring Food Safety Plans. In addition, the FDA recognizes “that ensuring the safety of the food supply is a shared responsibility among many different points in the global supply chain for both human and animal food,” so manufacturers may not be the only ones liable in many cases.7 The problem arises when manufacturers miss foreign contaminants or if foreign biological contaminants affect the integrity of the packaging.
Even if companies take the necessary steps, incorporate a HACCP plan and are vigilant, contamination can, unfortunately, happen at any time to any manufacturer. Using a third-party partner as an outside resource for foreign material inspection is important. Choosing a third-party partner with the experience, certifications, technology, processes and reputation to protect your brand is critical. Manufacturers can validate their internal processes and data using reports from their third-party inspection partner more quickly than they could internally.
Food packaging and the potential liability involved can be daunting. Still, with proper processes and procedures in place, manufacturers can have confidence that their products are hitting the shelves with a low probability of recall or lawsuit due to a packaging issue. While there is always a chance of foreign material contamination, quality packaging materials, quality assurance processes and quality third-party inspection partners can significantly reduce a company’s potential liability.
References
- Baraldi, L. G. (March 9, 2018). “Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: evidence from a nationally representative cross-sectional study.” BMJ Open.
- FDA. “FSMA Final Rule For Preventive Controls For Human Food”.
- Lusk, J. (October 15, 2019). “Consumer Beliefs About Healthy Foods And Diets.”
- Cheung, K. H. J. L. (2020). “Meet the 2020 Consumers Driving Change“. IBM.
- FDA(August 14, 1997). “HACCP Principles & Application Guidelines.”
- Hansen, C. “The Business Case For Reducing Food Loss and Waste.” Champions 12.3.
- FDA. “Food Safety Modernization Act (FSMA).”