The TWI: 2018 Training Management System Standard addresses various aspects of an organization’s Management System and identifies, explains and standardizes some of the best-known practices for ensuring that supervisor-to-operator relationships are strong and true. It also confirms that all training is performed in the most cost efficient, effective and safe manner possible. The standard provides guidance and tools for companies and organizations that want to ensure their products and services consistently meet customer’s requirements, while the quality remains top-notch.
TWI: 2018 sets out the criteria for an organization to integrate TWI practices into their management system and, when implemented properly, is the standard that can be certified (this is not a requirement). It can be used by any organization, large or small, regardless of its field of activity. It is equally as effective in service companies, as manufacturing or healthcare.
Read Part I: How training within industry empowers employees & facilitates continuous improvementThis standard is based on several quality management principles including a strong customer focus, motivation and implication of top management and process approach and continual improvement. Using TWI: 2018 helps ensure that customers consistently get good quality products and services, which in turn, brings many business benefits, and significant return on investment.
The adoption of TWI and the associated training modules into an organization’s management system must be a conscience decision and embraced as a business management strategy by the senior management team. TWI helps the organization see meaningful overall performance improvements and ensures the sustainability of the initiative.
Traditionally, the potential benefits of implementing TWI into their management system based on this international standard, are measured using the following Key Performance Indicators, (KPI):
- Increased Productivity
- Reduced Training time
- Reduced Labor-hours
- Reduced Scrap
- Reduced Grievances
The TWI: 2018 Training Management System International Standard can be used by internal and external parties. The standard’s requirements are intended to be complimentary to the organization’s current management system for product and service realization.
This standard employs the process approach, which incorporates the Plan-Do-Check-Act, (PDCA) cycle, as well as, risk-based thinking. This Process Approach enables an organization to plan its processes and their interactions.
The PDCA cycle enables an organization to ensure that its processes are adequately resourced and managed, and that the opportunities for improvement are determined and acted upon.
Risk-based thinking enables an organization to determine the factors that could cause its processes and management system to deviate from the planned results. It also allows a company to put preventive controls in place to minimize negative effects and to make maximize opportunities as they arise.
As it all comes down to working with and through people, it is imperative that the development of an organization’s personnel is adequately addressed. This includes, but is not limited to:
- Identifying and hiring the right people
- Appropriate and effective on-boarding of new employees
- New, as well as, refresher training
- Adequate Succession Planning
- Supervisor skills and knowledge training
- Effective problem solving
- On-going and effective safety training for a safe workplace
TWI training modules utilize a 4-Step Process to make the training consistent, standardized and easy to understand and comprehend. This process is time proven and meant to be rather rigid by design. The method looks much easier than it is to do, and requires, like any other skill, practice to perfect it. This standard DOES NOT include the actual methodology, but rather, documents the required elements of implementing TWI into business systems to ensure the integration of TWI into the organization’s culture and to achieve the highest return on investment. Appropriate TWI methods are identified throughout this standard.
TWI Training Management System Principles
This international standard is based on the quality management system principles described in the ISO 9001:2015 International System. It= is not intended to replace or supersede the ISO standard, but rather, to integrate with your current management system standard or GFSI-approved CPO. The focus is on enhancement by addressing the essential supervisor skills needed to “manage” the people aspect of doing business. By patterning it after the format of ISO, it is the intention of the TWI-Institute to make the TWI principles and training easier to integrate into an organization’s current management system.
The descriptions include a statement of each principle, a rationale of why the principle is important for the organization, some examples of benefits associated with the principles, and examples of typical actions to improve the organization’s performance when applying the principles.
The TWI Principles are based on The Five Needs Model for Good Supervisors:
- Knowledge of the work
- This refers to the kind of information that makes one business different from another (i.e.,materials, products, services, processes, equipment, operations, people, etc.)
- Knowledge of Responsibilities
- This refers to the organization’s situation regarding policies, regulations, rules, agreements, schedules, organizational structure, etc.
- Skill in Instructing
- This will assist supervisors in developing a well-trained workforce.
- Skill in Improving Methods
- This deals with utilizing materials, machines and manpower more effectively by having supervisors study each operation in order to eliminate, combine, rearrange, and simplify details of the job.
- Skill in Leading
- This helps the supervisor to improve his or her ability to work with people.
NOTE: Throughout this international standard, “Supervisor” is defined as anyone in charge of, or who directs the work of others. Therefore, “Supervisors can be identified by many titles: Supervisor, Manager, Foreman, Lead, Cell Leader, Director, VP, President, etc. “
The Process Approach
The TWI: 2018 – Training Management System International Standard promotes the adoption of a process approach when developing, implementing and improving the effectiveness of the management system and the supervision of people, to enhance customer satisfaction by meeting customer requirements and expectations.
Understanding and managing interrelated processes as a system contributes to the organization’s effectiveness and efficiency in achieving its intended results. This approach enables the organization to control the interrelationships and interdependencies among the processes of the system, so that the overall performance of the organization can be enhanced. Maintaining good employee relations, providing effective and efficient training, and making the daily habit of reviewing processes in order to continually improve them, must be integral to the organization’s culture and a primary strategic initiative.
The process approach involves the systematic definition and management of processes, and their interactions, to achieve the intended results in accordance with the organization’s policy statement and strategic direction. Management of the processes and the complete system can be achieved using the PDCA cycle (See Figure 1), with an overall focus on risk-based thinking aimed at taking advantage of opportunities and preventing undesirable results.
The application of the process approach in a training management system enables:
- An understanding and consistency in meeting requirements of necessary training
- The consideration of the processes of training, in terms of added value
- The achievement of an effective training process performance
- Improvement of training processes based on evaluation of data and information, (i.e., KPI’s).
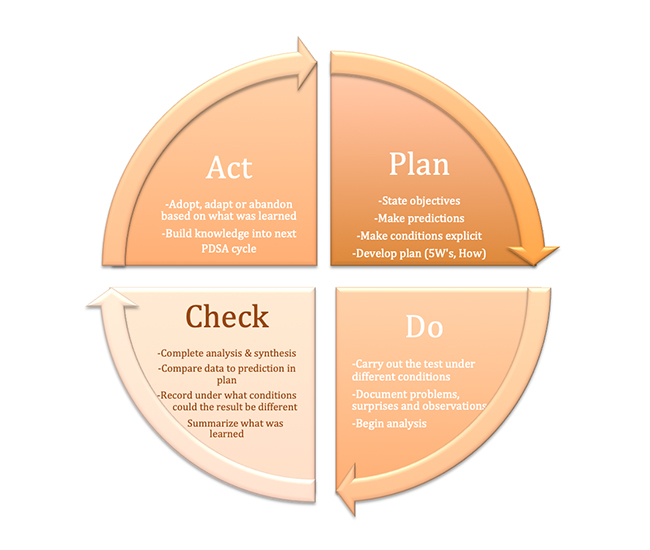
The PDCA cycle can be summarized as follows:
- PLAN: Establish the objectives of the system and its processes, and the resources needed to deliver results in accordance with customers’ requirements, (in this case, the employees of the organization), and the organization’s policies, and identify and address risks and opportunities.
- DO: Implement what was planned.
- CHECK: Monitor and (where applicable) measure processes and the resulting products and services against policies, objectives, requirements and planned activities, and report the results.
- ACT. Act to improve performance, as necessary to correct deviations, or to continually improve the processes. (Note: See Appendix A for an example of an implementation plan using a PDCA cycle.)
Risk-Based Thinking
Risk-based thinking is essential for achieving an effective management system and the proper training and development of the people. By using Risk-based thinking, management can carry out preventive actions to eliminate the potential negative effects of an unforseen occurrence (i.e., untrained personnel trying to complete a task, action or process step).
To conform with TWI: 2018, an organization needs to plan, implement and measure actions in order to address risks and opportunities associated with the training and development of the people. Addressing both risks and opportunities establishes a basis for increasing the effectiveness of the management system, including training and developing people, achieving improved results and preventing negative effects.
Relationship with Other Management System Standards
The TWI: 2018 – Training Management System International Standard is intended to allow seamless integration into an organization’s existing management system. To meet that end, this international standard was patterned after the ISO 9001:2015 International Standard since it is universally accepted, as setting the bar for management systems. Note: While the international standard is patterned after the ISO 9001:2015 International Standard, it is not dependant on compliance to that, or any other, management system format.
TWI: 2018 may, however, be implelented as a stand-alone training management system and does not include requirements specific to other management systems, such as those for environment, food safety, financial, or occupational health and safety management.
Summary
Virtually every process or problem has a human element as part of the equation. Unfortunately, we continually find that some organizations fail to connect the logic of TWI and its underlying principles to the actual daily realities of running the operation. If you don’t continually manage and practice the process, then it won’t sustain itself in the long run. The key to sustaining programs is to get off on the right foot with a solid plan. If you create a plan emphasizing the activities discussed above, along with problem solving and strong daily operational management, we know you’ll have greater success and increase your odds of sustaining an effective and efficient training program that will yield a significant ROI.
Resources
- ASQ/ANSI/ISO 9001:2015. Quality Management Systems-Requirements. ASQ. Milwaukee, WI.
Newslow, Debby. Food Safety Management Programs Applications, Best Practices, and Compliance. CRC Press. C2014. - TWI: 2018, Training Management System International Standard. The Central New York Technology Development Organization (TDO). Liverpool, NY.