This year is being described as “the year of FSMA compliance,” as many compliance dates for the various FSMA rules fall in 2017. As one might expect, the FSMA law and rules include many aspects of the established Global Food Safety Initiative (GFSI) standard; however, there are also differences in how they are applied to create better food safety enforcement.
At the most basic level, GFSI is an industry conformance standard for certification, while FSMA is a compliance regulation that must be met. However, both work together to ensure companies are effectively managing food safety.
GFSI Conformance
The GFSI is facilitated by the industry network of The Consumer Goods Forum. It provides a very solid foundation and supporting objective of “safe food for consumers everywhere”.
GFSI was originally established based on a growing pattern of food safety outbreaks throughout the international marketplace. This led to the proactive development of GFSI standards as an alternative to the more limited and less effective customer audits in place at the time. An important part of this outcome was that CEOs in the food industry—not a regulatory body—determined the need to address food safety through the GFSI food safety standard.
With its beginning as a benchmarking organization, GFSI has since evolved throughout the food supply chain as a strong means for achieving global food safety. It is now established, growing, and improving across the primary supply chains within the global food market.
As such, much work to address food safety has been accomplished by GFSI over the past sixteen years. In fact, the industry-driven aspect of GFSI along the food supply chain has led many companies to achieve levels of food safety comparable to those required to achieve FSMA compliance. Based on its collaboration of food safety experts, GFSI provides for a significant evolution of food safety programs and supports those requiring FSMA compliance.
FSMA Compliance
During a similar timeframe, the United States identified food safety as a major concern for the public. In the 1990s, a growing number of food outbreaks from biological contamination continued to spread, prompting the addition of controls within both the USDA and FDA. These brought the mandated requirement for Hazards and Critical Control Points (HACCP) and supporting Good Manufacturing Practices (GMPs) to specific industry sectors. However, these efforts were measured to have limited effect, as the outbreaks continued.
By the early 2000s, the public concern for food safety continued, and the FDA was determined to make changes. Along with Congress, the FDA commissioned research into the underlying issues that were resulting in the growing number and severity of food outbreaks. This research was being conducted and analyzed just as GFSI was determining its final group of benchmarked standards. At the same time, GFSI was positioned to be advanced into the U.S. market by food industry leaders, including Cargill, McDonalds, Walmart, Kroger, Coca Cola and Wegmans.
The outcomes from the FDA studies determined that the GMPs (in existence for the past 40 years) were not effectively implemented across the U.S. food industry. Further, the studies indicated that the ability to prevent food safety issues through specific controls would provide a means for reducing the number of foodborne illness.
This effort led to the development of FSMA, which passed in January 2011. Additional FSMA rules have since been published, starting in September 2016. The FSMA rules represent a rewrite of the existing FDA food safety regulations. However, with the FSMA law taking several years to roll out, the existing FDA laws remain in effect until they are replaced. These actions expand the FDA’s jurisdiction now and until full compliance of FSMA.
Bringing GFSI and FSMA Together
The presence of GFSI in the United States, as well as the GFSI certification of many suppliers to U.S. food importers, provides for a synergy between the GFSI standard and the FSMA law being enforced throughout the United States and its foreign suppliers. GFSI’s global focus provides the structure to adapt and meet many of the FSMA requirements, with the ability to expand to all FSMA requirements.
As one would expect, the FSMA law and rules include several aspects of the GFSI standard; however, there are many differences in how each is applied to encourage better food safety enforcement that must be considered. For instance, GFSI has the advantage of providing the time to develop programs, and thousands of companies are certified to the various programs under the standard. Conversely, FDA is implementing FSMA compliance over several years, with 2017 being a big year for compliance (based on the rules’ published dates, company size and industry segment).
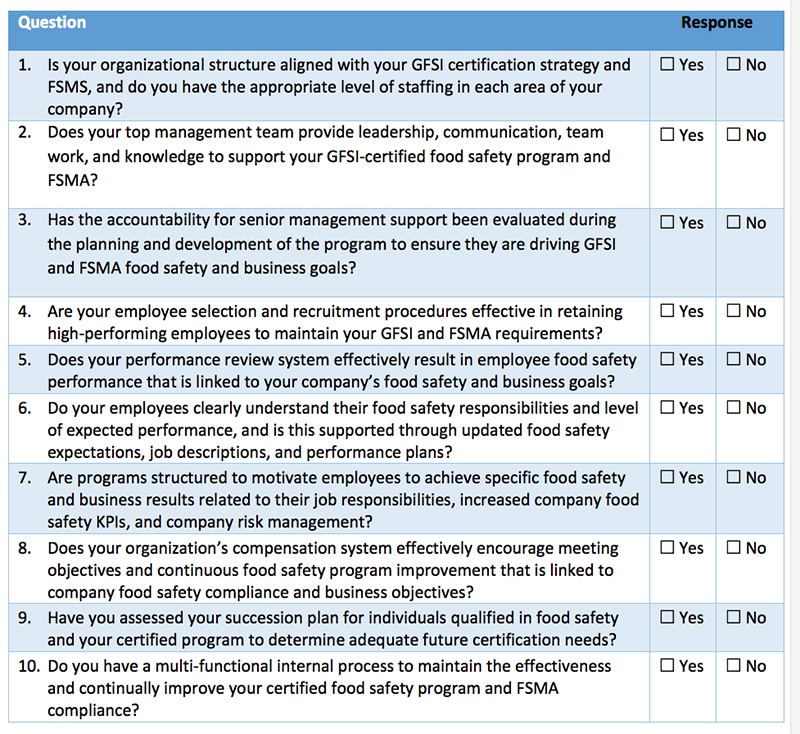
In this new order of food safety in the United States, those companies that have achieved GFSI certification should have an advantage over those who do not, provided they can align their GFSI programs with the FSMA law requirements. There is also a benefit to starting with FSMA and moving to a GFSI certification.
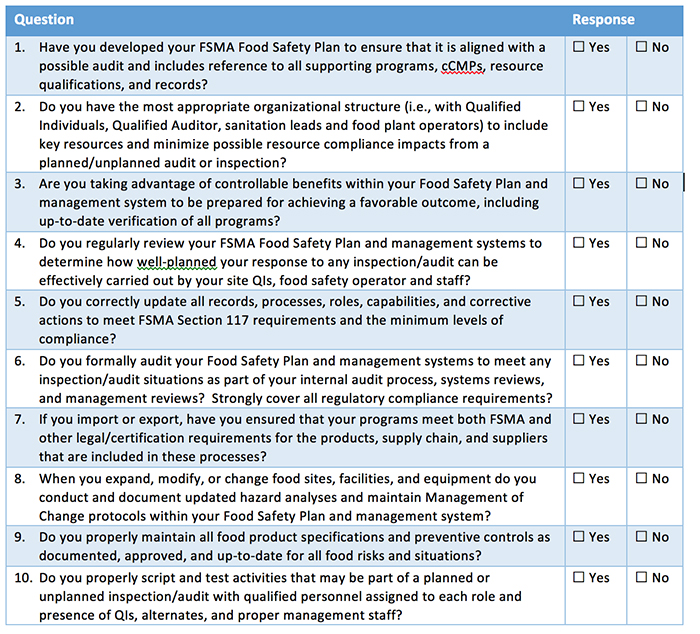
Existing GFSI certifications provide an established framework, with many of the program requirements similar to those required by FSMA. For example, personnel are required by both to establish HACCP and Food Safety Plans, as well prerequisite procedures (PRPs) and current-Good Manufacturing Practices (cGMPs). The challenges are ensuring the complete development of these food safety procedures to guarantee they meet both GFSI and FSMA requirements.
As another example, personnel requirements are similar but different under FSMA and GFSI, which calls for training, updating and qualifying resources. Ultimately, advanced HACCP training under GFSI provides the means for establishing a Qualified Individual under FSMA, but it requires expanding the training to include FSMA Preventive Controls and procedures. The resulting plan is the food safety plan that can be based on HACCP but with the proper additions to meet FSMA requirements.
Global Food Safety Conference
The upcoming Global Food Safety Conference (February 27 – March 3 in Houston, Texas) provides an opportunity for those seeking compliance to FSMA or certification to a scheme within the GFSI Standard to get a deeper understanding of food safety. With 2017 being the year of FSMA compliance, it is very appropriate that the Global Food Safety Conference be held in the United States this year. The conference will provide U.S. companies attending, as well as foreign supplier of products to the U.S. market, an educational opportunity and forum to reach out to experts from industry, government, and academia to better understand these two key areas for food safety program development. Some of the topics to be addressed at the conference include the following:
- Food safety management commitment and corporate governance
- Required training of food safety roles, including management, staff and operations
- Specific requirements of the documented food safety program or written programs under FSMA
- FDA requirements of the past and existing requirements prior to FSMA and the relationship of these as comparable to GFSI
- Implications for FDA enforcement under FSMA of these previous requirements and program requirements that may need to be formalized under FSMA
- The proof of evidence with supporting records required by FSMA that may be addressed in part by existing or GFSI-level food safety programs
- How to adapt a FSMA-level food safety plan and preventive controls cGMPs from existing programs, including GFSI, or develop these to function with existing programs
- Levels and numbers of qualified individuals, qualified auditors and competent sanitation for oversight and management of FSMA food safety plans
- Management reanalysis and update of the written FSMA programs to ensure compliance and readiness for inspection by FDA FSMA investigators
- Process used to ensure compliance with FSMA Preventive Controls and the other FSMA rules being issued in 2017 and 2018, including Foreign Suppler Verification, Sanitary Transportation and Intentional Adulteration
Kestrel has been a long-time advocate of GFSI, performing site certification program development support for hundreds of companies. We have served as a GFSI Stakeholder, Technical Working Group participation, and panelist at previous GFSI Global Food Safety Conferences. We look forward to seeing you at the 2017 GFSI Global Food Safety Conference and to helping you navigate GFSI conformance and FSMA compliance requirements.