As industry awaits next month’s final rule on preventive controls for animal food, companies in the animal feed business must be prepared for the changes, especially as it relates to having an aligned system with HACCP principles. In a Q&A with Food Safety Tech, Victor Muliyil, food technical project manager at SGS SSC North America, and Mary Williams, a quality assurance and regulatory affairs expert at Land O’Lakes, Inc, discuss where companies should be looking for gaps in their systems.
Food Safety Tech: What critical changes does FSMA introduce to the animal feed industry?
Victor Muliyil: FSMA introduces the primary change that all feed manufacturers must have a feed safety hazard control program that is in line with HACCP principles. Hazards likely to occur must be identified and controls implemented; and [although] hazards related to medications and prohibited material must still be controlled, the responsibility is on the manufacturer to identify all hazards and controls. The focus is on prerequisite programs, not just on critical control points.
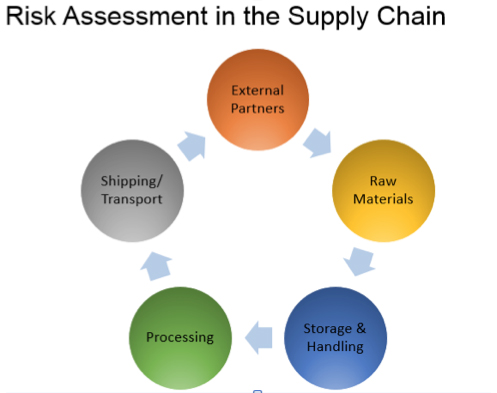
In addition, feed industry recalls can now be mandated by FDA, not just recommended. HACCP certification is not mandated by FDA, but several feed and food industry customers are looking for competent independent audit and certification of feed safety control programs. Trained internal auditors are required to verify the system. Traceability is required to the next level of distribution, as well as backward to key ingredients such as medications.
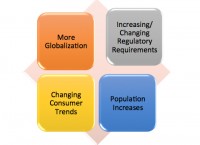
Mary Williams: Food industry leaders must now show they have “planned to work safely,” and this plan must be written down with documented evidence of training. This is a fundamental shift in approach, as FSMA indicates that all feed manufacturers must control feed safety hazards consistent with principles many of us have learned in HACCP. This speaks to prevention vs. reaction, so the prerequisite programs as a foundation must be in place first. This is a time of unprecedented change in the U.S. Food/Feed industry plus global supply chains that are expanding. While it is widely accepted that zero risk is unattainable, the approach that companies take to prevent having an issue, and to prepare for efficient and effective response in the event of a problem is seen as critical.
Product Safety Culture must be leadership driven and reinforced and furthermore, a strong product safety culture is a “choice”. Leaders of an organization set the tone and must proactively reinforce the expected outcome because it’s the right thing to do, not just because it is the newest food safety law.
While many feed companies are moving toward HACCP certification, it is not mandated by the FDA. Regardless of whether you build a HACCP plan or a Food Safety Plan, it is important for feed/food companies to start now. The cGMPs, new GMPs and most FSMA requirements are generally understood thus having more time to live and practice the programs implemented allows time for adjustments.
FST: Regarding GFSI certification, in what areas are companies in the animal feed industry the most under-prepared?
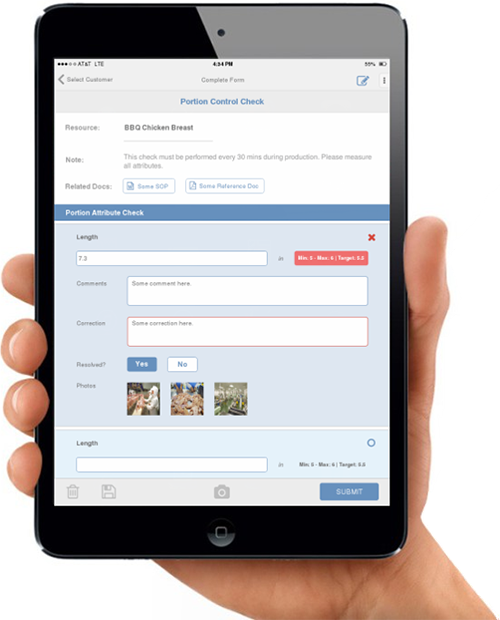
Muliyil: Management commitment, understanding and communication are key. Better training is needed to understand feed industry specific hazards and realistic controls. Currently, internal auditing is not very thorough and must be more structured. Corrective actions are not followed through to gauge effectiveness and are often not documented in adequate detail. Finally, validation is not well understood, nor is there specific guidance on this topic.
Williams: Management does not always clearly understand the need and requirements of “Management Commitment”. It requires active and visible participation at all levels of management. Managers must “walk the walk” and “talk the talk”. It may also require an investment in resources such as staffing, capital improvements, and training, to name a few. Management commitment is essential to support the development of a strong product safety culture. Failures in product safety culture increase the potential risk of outbreaks and deaths from foodborne illness.
The skills needed in the industry to meet these new expectations are different than what we needed before. It is not enough to just adopt new standards. We have to train and educate those who implement them.
We need to train for behavior – what do we want the trainee to be able to do? The training needs to be clear and practical. In addition, we need to educate for increased knowledge across the employee base. Don’t just send the managers and supervisors to HACCP class or auditor training, make sure we educate a multi-disciplined team including production employees.
Continuous improvement is an everyday concept and involves having a strong corrective action/preventive action program. Often deficiencies are corrected quickly, but not prevented over the long term, and this requires increased due diligence.
FST: Are companies with FSSC 22000 certification more prepared for the preventive controls rule?
Muliyil: FSSC 22000 is one of the GFSI benchmarked schemes that offer effective integrated food safety management, covering:
- Specific controls and scheme criteria for animal feed and pet food
- Global buy-in and adoption by many of the world’s leading feed and food manufacturing companies
- A top-down focus, including defined roles for management, requirements for policies and regular management review
- Prerequisite programs focused on hazard control, in line with HARPC and FSMA
- The HACCP system approach to structured food safety control, focused on medications & prohibited material control
- Traceability from suppliers through to customers
- Communication:External: Consumers, customers, service providers, suppliers, associations and regulators.
- Internal: Within a company and between all elements of the system
- Internal audit of the entire food safety management system and follow up
- Regular system updating to maintain rigor
Williams: A company certified in FSSC 22000 or one of the other GFSI benchmarked standards has implemented Codex HACCP and hygiene principles in their foundation programs. These same HACCP and foundation programs overlap with the requirements in the preventive controls rule and will support compliance to FSMA. It will be important to review all the FSMA requirements to ensure all elements are effectively covered in the current company program.
FSSC 22000 requires annual recertification and an annual self-audit. These two elements of review ensure that internal and external eyes are always looking for program compliance before a failure occurs. These are solid “prevention” elements that support FSMA compliance as well.