In a recent webinar, Robert Garfield, Senior Vice President of the Safe Quality Food Institute talked about the SQF standard, changes made in 2014, what is expected in 2015, and how companies can use SQF to be better prepared to comply with rules proposed under the Food Safety Modernization Act. We present below some excerpts from the webinar, organized under the 2014 GFSI Leadership Series. The next webinar in the series will focus on FSSC 22000. Click here to register.
Where is SQF going in 2015 and beyond?
Garfield: The standard is going to be focused on enhanced compliance programs and improving the database reporting systems. For instance, if it concerns someone in the bakery or dairy industry, we would like to know how they are doing versus the industry as a whole. We are hoping that better database reporting can help with this, especially when it comes to non-compliances.
Another area we are working on is establishing Cooperative Agreements. In 2014, we finalized an agreement with the American Feed Industry Association and we are working with their food safety program. We are hoping to not just work cooperatively with the private sector, but also with various government agencies and other stakeholders.
Other areas we are growing in 2015 are expanding our language alternatives, subject matter training and developing industry specific guidance.
There are many changes proposed to food safety regulations and food safety schemes such as SQF. How will companies be affected by these changes and why is embracing these changes so important to industry?
Garfield: Embracing all these changes is critical for the food industry to do everything they possibly can to ensure that they are making and selling a safe product. At the end of the day, there is no one ‘magic bullet’ solution to food safety. Embracing these changes to food safety rules and standards will help the CEO and management team sleep better at night, knowing that they are doing what they can to protect their product, their brand name and their consumers. Also, companies need to understand that the regulatory climate will completely change in the next few years, so it’s critical for companies to start acting now to meet these new requirements that will start being in effect from October 2015.
How can companies start preparing today for tomorrow’s SQF?
Garfield: I tell companies and retailers I talk to that if they are interested in doing SQF because they want to be ‘GFSI certified,’ that’s the wrong reason to do this. To get started, management commitment and changing the culture of the entire company is critical. Starting from the CEO and going all the way to the man operating machinery on the floor, you should aim to get a commitment to food safety, where food safety management is the most important issue for the company. If you start working on that today, you can accomplish great things for the company in not just reducing recalls, but improving the overall functioning of that company.
How can SQF help prepare companies for FSMA?
Garfield: The first step is to look at the Preventive Controls and the Fresh Produce rules and see how these apply to your company. I suggest hiring an independent expert to take a look at your facility and see how your company fares against these rules and have a better understanding about where you will be when these rules are finalized by October 2015. While you will have one to three years to comply with these rules after that point, you need to get the management buy in and strong food safety management systems in place now. Start now, and don’t wait for the final rules to be announced.
Listen to this complimentary webinar today to learn more about how SQF differs from other food safety programs, unannounced audits, changes with allergen control standards, and how to become SQF certified. Click here to access the recording.
2014 GFSI Leadership Series continues with FSSC 22000: The Road Ahead. Click here to register for this informative webinar on Friday, September 26, 2014, featuring Jacqueline Southee, U.S. Liaison, FSSC 22000, who will talk about what’s new for FSSC 22000 this year, where FSSC 22000 is going in 2015 and beyond, how you will be affected by the changes, and how to start preparing today. Plus Jacqueline will take your questions live!






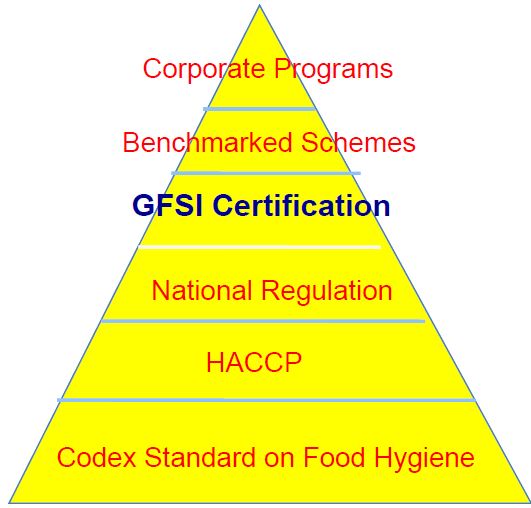

 At the very base of our efforts that are ensconced within these guidance on a sector by sector basis, are the international standards of science based within the Codex Standard on Food Hygiene. On top of that are Hazard Analysis and Critical Control Points or HACCP standards. Above HACCP are National Regulations, which includes FSMA in the U.S. and the Safe Food for Canadians Act in Canada. In Europe it’s something different, in Japan it’s something different, but all have iterative levels of science-based regulation in place to ensure the safest control of the food and management of the food. Above National Regulation is GFSI Certification.
At the very base of our efforts that are ensconced within these guidance on a sector by sector basis, are the international standards of science based within the Codex Standard on Food Hygiene. On top of that are Hazard Analysis and Critical Control Points or HACCP standards. Above HACCP are National Regulations, which includes FSMA in the U.S. and the Safe Food for Canadians Act in Canada. In Europe it’s something different, in Japan it’s something different, but all have iterative levels of science-based regulation in place to ensure the safest control of the food and management of the food. Above National Regulation is GFSI Certification.