The FSMA HARPC regulation has been in the implementation phase for approximately a year. Many small and entrepreneurial businesses are in the process of starting or finalizing the development of a food safety plan to comply with FSMA requirements. This includes program development, operational awareness and employee training. Often, small companies find this development more challenging compared to mature companies for several reasons, including a lack of resources or simply not knowing where to start.
The following eight tips can help small businesses that are developing a food safety plan to comply with FSMA.
1. Don’t be scared.
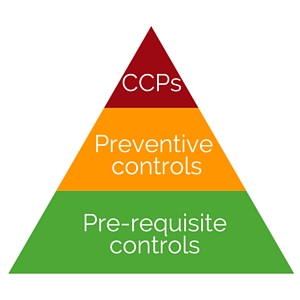
FSMA Preventive Controls is nothing scary. It is simply a series of food safety protocols and related documentation. It might seem overwhelming at the beginning with many documents and changes; however, it is actually a good method and tool to help strengthen operation lines and management.
FSMA helps businesses sustain and streamline processes. It is helpful to first map out the production process from the very beginning (when raw materials are received) through the end (when finished products leave the facility). The more details that are documented on the process, the easier and less time consuming it will be later to prevent potential risks.
2. Be familiar with the process and the FDA hazard types.
Once all processes are mapped, take time to study and get familiar with them. It will be helpful to have a team of individuals with different job functions review process maps together. The objectives are to identify the following:
- Where is the weakness?
- Where can weaknesses be controlled?
- What should be monitored?
- When is a good time to monitor each process step?
According to FDA, five hazard types need to be considered and prevented: Physical, chemical, biological, intentional adulteration and radiological. These five types should always be kept in mind when reviewing and analyzing the direct production and non-direct production processes.
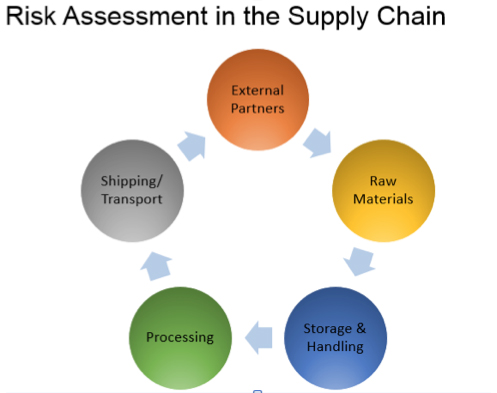
3. Thoroughly understand the entire supply chain.
Supply chain management is one of the key preventive controls required by FSMA. Just like mapping out the process, FDA requires each business to have a thorough understanding and control of its supply chain to ensure the risks are minimized from raw materials to end consumers. Whether you have foreign suppliers, distribution centers or co-manufacturers, finished product safety must not be compromised by any party. If foreign suppliers are being used, FSVP (Foreign Supplier Verification Program) must be implemented and communicated to vendors.
4. Think in food safety mindset.
If your business has just been established, then congratulations! You have the opportunity to start everything right from the beginning. Take food safety into consideration throughout every step in the process and operation. Considering food safety aspects and preventing hazard types might help you make your next good business decision.
5. Get everyone involved!
Food safety is not only the food safety and quality departments’ responsibilities; it reflects the entire company’s operational structure—from building structure, security, production line, and supply chain to procurement, HR and finance. Get everyone involved, from top management to line workers. Their expertise, experiences and feedback will help the entire program’s implementation and execution. With the inputs from each department function, the food safety program will be more practical to the entire business operation and, therefore, will be more solid and sustained, especially when it comes to ongoing implementation.
6. Designate one project leader.
If FSMA program development is considered a project that the whole company engages in, a project leader is required to make the journey efficient and smooth. The leader needs to have both the company operational experience, as well as food safety knowledge. The leader plays an important role in leading the project, coordinating the timeline, prioritizing work across departments, and communicating with all levels of employees.
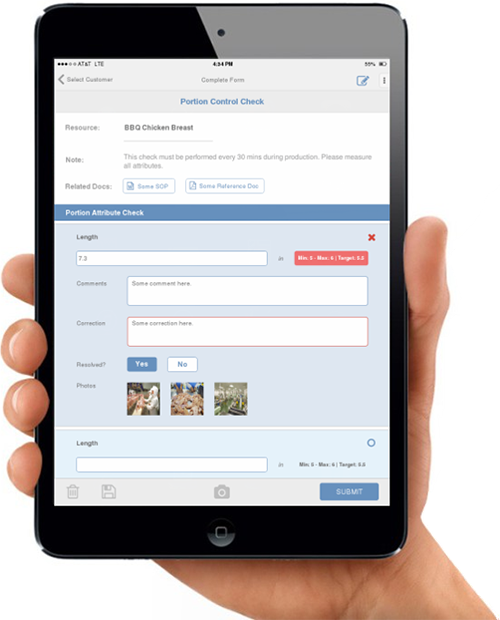
7. Keep everything documented and recorded.
Documentation and recordkeeping are core to the entire program. Say what you do by writing down all procedures, policies, programs and SOPs. Do what you say by demonstrating what is contained in all records kept onsite. This is not only for audit purposes, but also for your own business growth. Your own operation data is the best data to improve and modify your processes, if needed. Records can be used for trend study and analysis after years in business. Records can reveal whether methods or programs implemented are working effectively and helping the business. Records can also provide strong support/evidence when there is an unexpected event.
8. Utilize free third-party resources.
There are many technologies linking the entire world together—leverage them to learn from your peers. GFSI-recognized certification programs, such as SQF, FSSC22000 and IFS, are releasing a global market program to specifically help small business start their programs. Webinars and trainings are available on many program development and food safety hot topics to help address challenges, and there are many tools and templates available for download to assist with documentation and recordkeeping.
Although there are a lot of perspectives and aspects to be considered to comply with FSMA, compliance can be achieved one step at a time. Start by mapping out your own production process today.