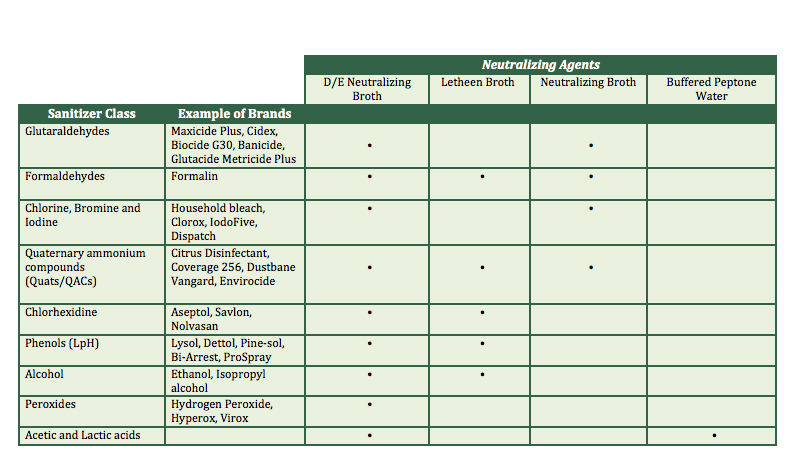
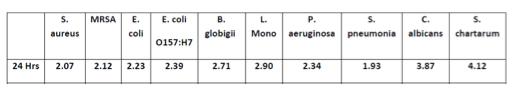
Today food companies will have access to a new whole genome sequencing (WGS) test that could help them prevent dangerous pathogens from getting into their products. Released by Clear Labs, the test takes a detailed approach to identifying pathogen strains in samples, providing information about their geography and from which food groups they originate.
In an exclusive interview with Food Safety Tech, Mahni Ghorashi, co-founder of Clear Labs, explains how he expects the company’s new test, which has a five- to seven-day turnaround time, will offer companies with a more accurate yet less expensive alternative to protecting consumers by actively monitoring their supply chain for emerging pathogens.
Food Safety Tech: What differentiates this WGS test from current available solutions?
Mahni Ghorashi: No one has been able to provide the food industry with modern whole genome sequencing techniques for food safety. What we’re releasing is a quantum leap in terms of what’s been available on the market today. Whole genome sequencing has been largely siloed to regulatory bodies like FDA and CDC to trace outbreaks and inform investigations—the technologies and techniques that they’re using are actually fairly old; they’re some of the original WGS techniques that emerged on the next-gen sequencing platform. We’ve taken the most advanced techniques on the NGS platform for human disease exploration and personalized medicine and adapted them for food industry.
What gives our WGS test a competitive advantage over legacy-based methods is two fold:
1. Clear Labs has a 2-million+ entry-curated database of genomic information and sequences for the accurate ID of food ingredients (pathogenic organisms and microbiomes). Its accuracy and the confidence level that comes behind our matching is a huge step above anything that’s available in the public domain today.
2. Being able to place pathogenic strain information in the context of overall food ingredients and samples. The whole genome sequencing test we developed has been specifically catered for the food industry, and for food samples in particular, [versus] FDA’s GenomeTrakr, CDC’s PulseNet, or other food safety labs that are offering full genomic sequencing of pathogen strains—they’re using some of the earliest methods to do this. On the NGS platform, we’re able to put those strains in the context of food ingredients and suppliers: Specifically, [matching] bacterial strains with food ingredients [and] suppliers.
FST: Does this test target specific foods?
Ghorashi: Our platform particularly shines in complex foods. The value of next-gen sequencing and DNA barcoding over PCR-based technologies, which is the gold standard in food safety, is its stability to break down complex food ingredients into all of their known parts, and to look in a universal and unbiased way into food samples. It’s untargeted, so you don’t have know what it is that you’re looking for—and that’s the real power.
FST: What impact do you anticipate for this test, especially in the context of FSMA?
Ghorashi: Our customers are using [the test] for monitoring ingredient supplies and the effectiveness of preventive and sanitary controls [and] to match specific pathogen strains to specific food ingredients. They are using it for proactive testing for FSMA compliance—there’s a lot of movement in this direction and hefty budgets are being allocated to put new preventive controls in place in response to FSMA; whole-genome sequencing will play a big role, and we anticipate large-scale partnerships with agencies and private industry on that front. And the most obvious use case is that it’s being used for techniques to mitigate or reduce the risk of product recall and outbreak.
We’ve been able to significantly reduce the price point on whole-genome sequencing, and all of our tests across the board, because we’re intimately familiar with how the inner workings of these platforms and how to best optimize them for scale and cost efficiency. We think the test will be more accurate and leaps and bounds ahead of what’s available, as well as cost competitive. We’re excited about the work we’re doing and its impact on food safety. I don’t think the food industry—retailers and manufacturers—have ever had access to these kind of tools and they’re being made available just in time for FSMA, as the industry moves towards a more proactive approach to food safety and [takes] preventive measures in their supply chains. Hopefully we’ll soon be living in a world where outbreaks, illness and the financial toll are a thing of a past.
| Clear Labs also just released a microbiome test that helps companies associate microbiomes with specific food ingredients.
Mahni Ghorashi: The microbiome test we’ve developed is able to sequence samples from the human gut and from food, and look at how the microorganisms are interacting. Our customers for this test have been large brands that have advanced R&D departments and academic research centers that are looking for how diet research and the microbiome interact together and how new product development can help us move toward personalized diets when it comes to prebiotic and probiotic diets.” The impact of the microbiome and the correlations between bacteria of the human gut and the bacteria in the food we eat. The prevailing thesis at the moment is that the microbiome has a significant impact on our health when it comes to disease risk and diet, inflammation and mood disorders. We’re seeing very forward thinking brands like Nestle, ConAgra and Mars putting a lot of attention on the impact of the microbiome when it comes the development of new products, [such as] prebiotics and probiotics, or even specific food products as it pertains to the microbiome. We believe that this intersection— nutrigenomics and the personalized diet—is going to be a massive market, and we’re at the early stages of that. |