In recent years, several food products typically considered safe by consumers have fallen victim to recalls as a result of Listeria monocytogenes (Lm). Caramel apples, ice cream, packaged salads and frozen vegetables were responsible for sickening dozens of people and killing more than 10. These products are part of an alarming group of common foods that have caused outbreaks, including milk, spinach, sprouts, peanut butter, cheese, cantaloupes and raw cookie dough. And the broad range of pathogens causing these outbreaks is just as diverse, and they continue to find creep into food processing facilities, finished food products and consumer homes.
At the 2016 Food Safety Consortium, Shawn Stevens will moderate the workshop session, Bringing the final FMSA pieces together: You have a basic preventative control program, what’s left? | Friday, December 9 | LEARN MORERegardless of sophistication or expertise in pathogen control, there isn’t a single company out there that is immune to the risk of contamination. Why? Well much of the foods (or ingredients) that we consume are grown and harvested in environments that are susceptible to contamination. Fruits, vegetables and other products, such as spices, can easily become contaminated with Lm, Salmonella or E. coli in the fields where they are grown, in transit or in the processing facilities.
Once pathogens are introduced into the processing environment, they can quickly spread and contaminate food products. Recent studies reveal that Listeria is a significant concern in these environments. For example, out of 5,000 samples from the food preparation areas of 30 retail grocery establishments, approximately 10% tested positive for Lm. These are scary numbers considering almost 16% of those who become infected with Lm will die.
In today’s new environment, FDA will be seeking justification to bring criminal charges whenever a contaminated product causes human illness. You should be nervous about this: If your company sells finished goods into commerce, those products may be selected for sampling and testing, and your company runs the risk that the results will come back positive for a pathogen of concern. And what’s more troubling is the fact that many companies do not conduct environmental testing in their food processing facilities, and so they have no idea whether pathogens (whether transient or resident) are lurking within their facilities. Thus, a microbiological profiling study conducted under the veil of the attorney-client privilege should be conducted to determine the presence of any microbiological persistence issues within a facility. Upon completion of the study, a company should invest in pathogen-reduction technologies to decrease the chances that FDA will uncover pathogens in the environment during an inspection. Finally perform a criminal protection audit to help strengthen company programs and develop protocols that will further protect against criminal exposure.
The bottom line is that if food companies do not take extraordinary measures to identify Lm in their facilities, perform a comprehensive investigation to find the root cause or source, and then destroy and eliminate it completely, the pathogen will likely persist and, over time, intermittently contaminate their finished products.
Microbiological Profiling Studies
Lesson number one from the Blue Bell Lm outbreak is that pathogens can be extremely elusive and, as a result, a simple environmental monitoring program will never save your company from being involved in an outbreak or being the focus of criminal sanctions. All food companies should be aggressively testing for Lm (or other pathogens, depending upon the product risk profile) in their facilities and must take strong action against sporadic or intermittent positive findings. Although many food companies view a single operational failure as the culprit of an outbreak, the reality is that in most cases, the cause is something far more subtle, far more persistent, and far more dangerous. In recent years, a large number of outbreaks have involved Lm and antibiotic-resistant Salmonella that was linked to products that had been processed over multiple months.
Food companies should conduct a comprehensive one-time microbiological profile for pathogens in their processing facilities. Be sure to coordinate your profiling study with a lawyer experienced in food safety to make sure that the study is designed correctly and that the results will be protected under the attorney-client privilege. Once the results are reported, the company can take care of any positive findings, identify the contamination source, implement technologies to reduce and control the contamination, and develop a microbiological control and monitoring program to ensure that the pathogen remains controlled moving forward.
Pathogen Reduction Technologies
The second lesson learned from the Blue Bell case is that, when Lm or any resistant pathogen is found sporadically in the environment, what was once regarded as effective corrective actions (i.e., re-cleaning, re-sanitizing and re-testing) are no longer enough. In addition to existing cleaning and sanitizing procedures, companies should use new pathogen reduction technologies to help control the environment.
Inexpensive air and surface treatment technology that sanitizes the food processing environment is now available. The treatment is approved for use in occupied spaces and provides 24-hour treatment of the environment. By using active air and surface treatment, food processing companies can gain a level of control and decrease the possibility that any pathogen, if introduced, will persist or establish a niche.
Puradigm, LLC, for instance, utilizes a multi- patented, NASA-based active air and surface sterilization approach to control pathogens in the food processing environment. In studies performed by Kansas State University, the company obtained a 2.9 Log reduction on environmental food contact surfaces in the food processing environment. Similar reductions for other pathogens are displayed in Table I.1
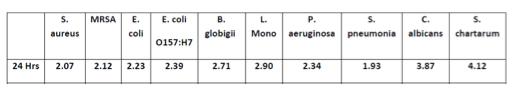
I make this observation because, given the risk created by the FDA’s war on pathogens, food companies should invest in technologies to better control pathogens in their food processing environments. Once these preventative technologies are put into place, companies can perform periodic microbiological monitoring to validate that the controls are effective and working as designed. If such solutions are employed, there is a greater likelihood that when FDA arrives to perform microbiological profiling, the agency will be less likely to find positive test results from the food processing environment, better protecting food companies from additional regulatory or criminal exposure.
Criminal Protection Audits
In addition to commissioning microbiological profiling studies in facilities and employing active air and surface sterilization technologies, food companies should also perform internal criminal protection audits. These audits should be designed to identify gaps in existing company protocols and develop written programs designed to help navigate the challenges posed by any food safety issues uncovered.
If developed correctly, the written program should provide the company with a decision-tree to follow in the event of a positive environmental finding, a series of customer complaints relating to the safety of a product, or a notification from a governmental entity of a potential food safety problem. These protocols and programs, if followed in the event of a food safety issue, can help ensure that the conduct of the company in response to any such issues will in all cases be appropriate, and that there will not be any basis upon which FDA or DOJ could support criminal charges.
Conclusion
The FDA (in cooperation with DOJ) has launched a war on pathogens. The agency is targeting food products at retail and engaging in microbiological profiling of all food companies. Unless companies act now to better quantify and control pathogens in the food processing environment, they are exposing themselves to incredible food safety risk, including significant brand damage (in the event of a recall) and criminal sanctions (if their product is linked to human illness). Companies must carefully consider the emerging risks facing them and take measures to decrease and eliminate their exposure.
Reference
- GC/MS Evaluation of Compounds in Air Samples in a Controlled Environmental Chamber Equipped with a Puradigm Advanced Technology Cell, November 5, 2013, Dr. James Marsden, Kansas State University Food Science Institute.








