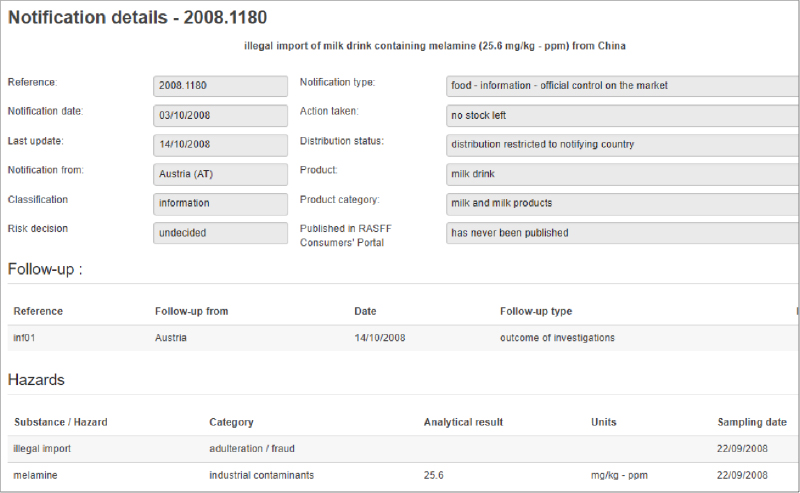
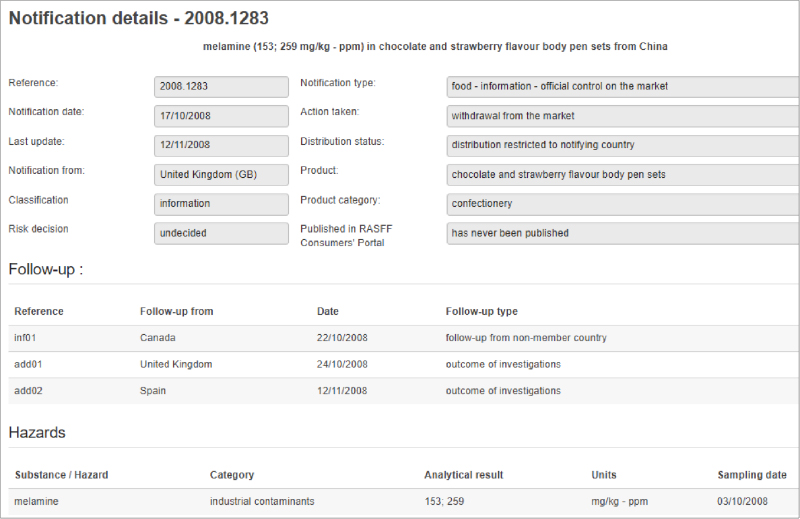
In 2011 three U.S. government agencies, the CDC, the FDA and the USDA’s Food Safety Inspection Service (FSIS) created the Interagency Food Safety Analytics Collaboration (IFSAC). The development of IFSAC allowed these agencies to combine their federal food safety efforts. The initial focus was to identify those foods and prioritize pathogens that were the most important sources of foodborne illnesses.
The priority pathogens are Salmonella, E. coli O157:H7, Listeria monocytogenes and Campylobacter. To research the most important product sources, the three agencies collaborated on the development of better data collection and developed methods for estimating the sources of foodborne illnesses. Some of this research was to evaluate whether the regulatory requirements already in effect were reducing the foodborne pathogens in a specific product matrix. The collection, sharing and use of this data is an important part of the collaboration. For example, when the FDA is in a facility for routine audit or targeted enforcement, they will generally take environmental swabs and samples of air, water and materials, as appropriate, which are then tested for the targeted pathogens. If a pathogen is found, then serotyping and pulsed-field gel electrophoresis (PFGE) fingerprinting is performed, and this is compared to the information in the database concerning outbreaks and illnesses. This data collection enables the agencies to more quickly react to pinpoint the source of foodborne illnesses and thereby reduce the number of foodborne illnesses.
The IFSAC strategic plan for 2017 to 2021 will enhance the collection of data. The industry must be prepared for more environmental and material sampling. Enhancement of data collection by both agencies can be seen through the FSIS notices and directives, and through the guidance information being produced by the FDA for FSMA. Some examples are the raw pork products exploratory sampling project and the FDA draft guidance for the control of Listeria monocytogenes in ready-to-eat foods.
Starting May 1 2017, the next phase of the raw pork products exploratory sampling project will begin. Samples will be collected and tested for Salmonella, Shiga-toxin producing E. coli (STECs), aerobic plate count and generic E. coli. In the previous phase, the FSIS analyzed 1200 samples for Salmonella for which results are published in their quarterly reports. This is part of the USDA FSIS Salmonella action plan published December 4, 2013 in an effort to establish pathogen reduction standards. In order to achieve any objective, establishing baseline data is essential in any program. Once the baseline data is established and the objective is determined, which in this situation is the Health People 2020 goal of reducing human illness from Salmonella by 25%, one can determine by assessment of the programs and data what interventions will need to take place.
The FDA has revised its draft guidance for the control of Listeria monocytogenes in ready-to-eat food, as per the requirement in 21 CFR 117 Current Good Manufacturing Practice, Hazard Analysis and Risk-Based Preventive Controls for Human Foods, which is one of the seven core FSMA regulations. Ready-to-eat foods that are exposed to the environment prior to packaging and have no Listeria monocytogenes control measure that significantly reduces the pathogen’s presence, will be required to perform testing of the environment and, if necessary, testing of the raw and finished materials. Implementing this guidance document helps the suppliers of these items to cover many sections of this FSMA regulation.
The purpose of any environmental program is to verify the effectiveness of control programs such as cleaning and sanitizing, and personnel hygiene, and to identify those locations in a facility where there are issues. Corrective actions to eliminate or reduce those problems can then be implemented. Environmental programs that never find any problems are poorly designed. The FDA has stated in its guidance that finding Listeria species is expected. They also recommend that instead of sampling after cleaning and/or sanitation, the sampling program be designed to look for contamination in the worst-case scenario by sampling several hours into production, and preferably, just before clean up. The suggestion on this type of sampling is to hold and test the product being produced and to perform some validated rapid test methodology in order to determine whether or not action must be taken. If the presence of a pathogen is confirmed, it is not always necessary to dispose of a product, as some materials can be further processed to eliminate it.
With this environmental and product/material testing data collected, it is possible to perform a trends analysis. This will help to improve sanitation conditions, the performance of both programs and personnel, and identity the need for corrective actions. The main points to this program are the data collection and then the use of this data to reduce the incidence of foodborne illness. Repeated problems require intervention and resolution. Changes in programs or training may be necessary, if they are shown to be the root cause of the problem. If a specific issue is discovered to be a supply source problem, then the determination of a suppliers’ program is the appropriate avenue to resolve that issue. Generally, this will mean performing an audit of the suppliers program or reviewing the audit, not just the certificate, and establishing whether they have a structured program to reduce or eliminate these pathogens.
Continue to page 2 below.