Dr. Kathryn Birmingham, one of ImEPIK’s PCQI training experts, provides guidance to Juan, a future PCQI in a plant that receives ingredients for ready-to-eat energy bars.
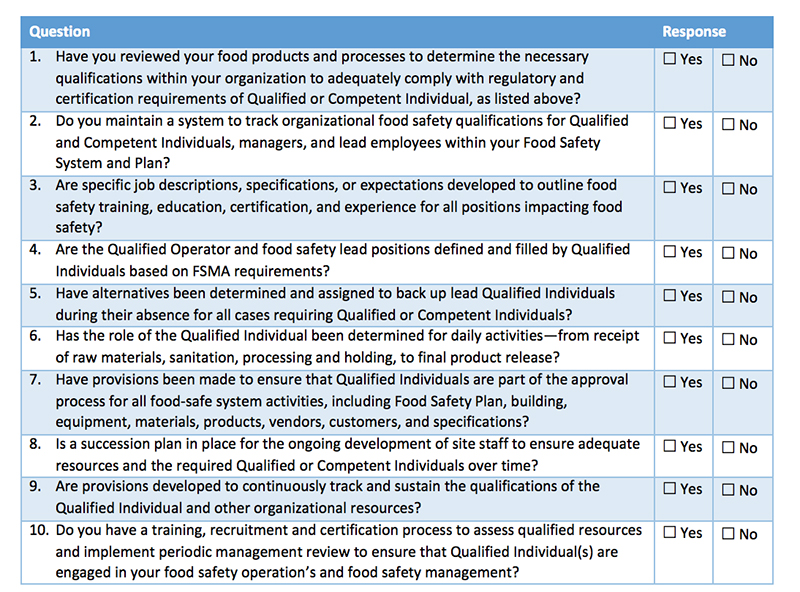
Juan: I’m new on the food safety team at a small company and the next person to be trained as a PCQI. Our team wants to make sure we are meeting the requirements in our food safety plan under the Preventive Controls for Human Food Rule in FSMA. There are a lot of players along our ingredients supply chain. Who is ultimately responsible for product safety?
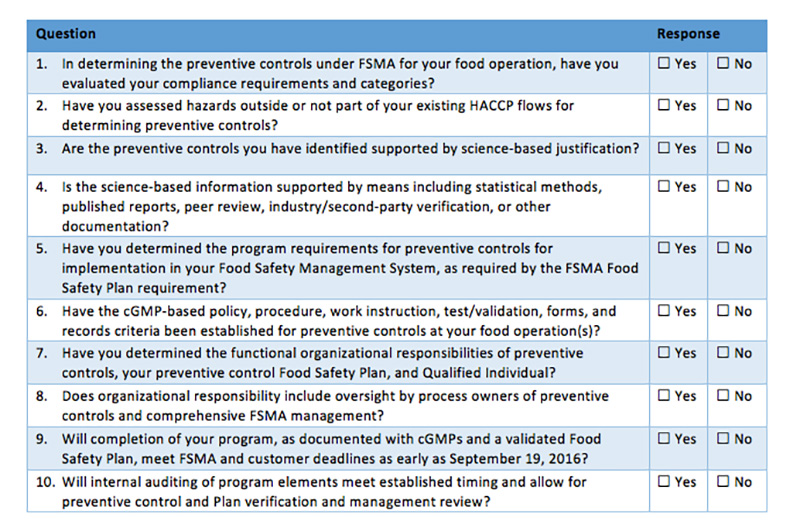
Kathryn Birmingham: As you know Juan, if you manufacture, process, hold or pack an ingredient or food product, food safety is your responsibility. For all of the players along the supply chain FSMA focuses on risk assessment and identifying hazards and preventive controls when required. Your team must have a plan and implement verification activities for the supply chain preventive controls for the food ingredients with hazards you have identified needing a control.
Juan: So, we are sourcing chocolate from a number of suppliers or our bars. They all provide COAs with the shipment that tell us the chocolate is manufactured to be free of pathogens like Salmonella. Usually we get a laboratory report on the sample testing for vegetative pathogens from the supplier for each shipment. We put that in our food safety plan to verify that the hazard was controlled by the supplier. But one of the suppliers has not provided sample testing results we requested. We have finished product to get out the door, but we have to ensure our product doesn’t harm consumers. On top of that, we can’t risk a costly product recall.
Kathryn: Right, Juan. That Certificate of Analysis may not be enough to verify that your chocolate supplier is effectively controlling for the hazard of Salmonella. For your product process flow the chocolate will never have a kill step to mitigate the hazard. If you cannot be sure that the hazard has been significantly minimized or prevented before receipt of the chocolate – per section 117.410 in the PCHF Rule – you have some choices to make. If you are using a foreign supplier there are considerations if the supplier is or is not in compliance with the FDA’s Foreign Supplier Verification Program.
Juan: So it looks like we may have to take on the cost and additional time of sample testing?
Kathryn: Remember, supplier approval is based on performance. If your supplier does not give you the evidence for verification you may need to conduct an onsite audit, perform sampling and testing and review other supplier records. You decide if the supplier meets your Supply Chain Control Program or Foreign Supply Chain Control Program.
Juan: My team members need to learn more about what we need to do to comply with FSMA and the PCHF Rule. Tell me about what we can learn through PCQI training.
Kathryn: Preventive Controls Qualified Individuals are trained in a methodical process for decision-making on hazards and preventive controls. The best training fosters a positive food safety culture and includes practice on team scenarios.
A PCQI must be able to identify hazards associated with a product and process, determine the appropriate preventive controls and develop associated monitoring and corrective actions for hazards that are identified. PCQIs must also establish and implement appropriate verification activities for the application of preventive controls. All of that is included in the food safety plan they oversee.
Juan: What choices do we have for online PCQI training?
Kathryn: First choose your food safety team members. If your company is registering with the FDA you are required to have at least one PCQI at each facility. Most companies train multiple or back up employees for the PCQI role to ensure they are covered during vacations, sick time, various shifts or employee turnover.
Look for courses that include the FDA’s standard curriculum, like ImEPIK’s PCQI Online. The PCHF Rule does not require that PCQIs hold a specific training certificate, but FDA inspectors want to see that the PCQI has been successful in a training with the requisite learning objectives and content. There are many PCQI training options on the market. Some providers claim that their training is the only accepted training – that’s simply not true.
Look for courses that have a multiple of scenarios with different food products and challenge situations for practice and wider breadth of learning.
ImEPIK’s PCQI Course is interactive and 100% online. The ten-module training is entirely self-paced thus does not require travel or scheduling on-line webinars or sessions. You simply log in, work through the course as you have time, and earn your completion certificate to document in your food safety plan. If you take a break, the work you have done will be saved, and you pick up where you left off when you return to the course. This allows for reflection and practice in the workplace as you move through the modules.
It’s an ever changing environment for the food safety professional and quality training makes a big difference in keeping up with changes and staying regulatory compliant. Take PCQI Online and position yourself and your facility for food safety success.
About Kathryn Birmingham, Ph.D
 Kathryn Birmingham, Ph.D., is Chief Operating Officer of ImEPIK. Birmingham leads the company’s course development teams and ensures that the online training solutions are of high quality. She is certified as a Lead Instructor to teach the FSPCA’s Preventive Controls Qualified Individual course.
Kathryn Birmingham, Ph.D., is Chief Operating Officer of ImEPIK. Birmingham leads the company’s course development teams and ensures that the online training solutions are of high quality. She is certified as a Lead Instructor to teach the FSPCA’s Preventive Controls Qualified Individual course.
Dr. Birmingham taught graduate and doctoral students at the University of Florida and served as Dean of Arts and Sciences at Florida State College. At the latter she lead the Biotechnology Degree program and Institute for Food Safety analytical lab. She was Principal Investigator (PI) for its National Science Foundation studies.
Content Sponsored by ImEPIK.