Note: FSMA will include the scheduled compliance inspection as part of the implementation of rules. This will occur in the next several years for many food companies.
With FSMA rules moving to the compliance stage, food companies must prepare appropriately to best respond to the requirements and, correspondingly, to additional inspections. These inspections are in addition to others, including GFSI with its emphasis on unannounced level audits for some schemes. For example, these audits may be required by the code (as with SQF) or as part of customer arrangements per certification contracts.
Learn more about FSMA Inspection Readiness at this year’s Food Safety Consortium in Schaumburg, IL | December 7-8, 2016 | REGISTERWith the growing potential for inspections and audits, a well-planned program and response must be developed, implemented and tested to achieve a most successful outcome. This is an important area to address, especially given the many changes in compliance under FSMA, greater scrutiny under GFSI, and a rapidly changing responsibility for food safety management resources.
For companies experienced with past FDA compliance audits, the new rules and Section 117 cGMPs will require more formalized programs and strong evidence of compliance through internal audits and oversight by Qualified Individuals (QI). The inspectors will look to focus heavily on new requirements and the “letter of the law”. Additionally, organizations under the Preventive Control Rule must have multiple Food Safety Plan QIs, qualified audit resources and competent sanitation management, along with competent plant operators. It is critical to have established roles, planning and testing as part of any inspection readiness program.
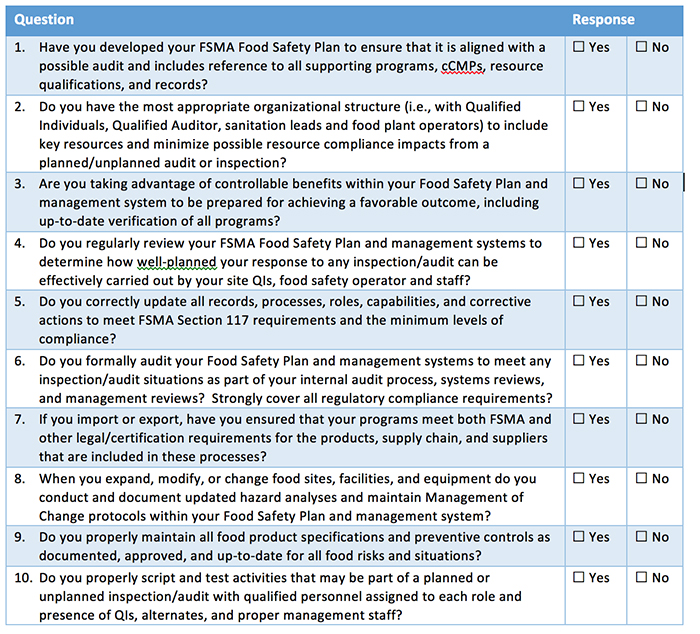
Self-Diagnostic Assessment Tool
The following self-diagnostic assessment tool can help organizations better determine their current state of planning when it comes to developing inspection readiness. To complete your own planning assessment, review your progress compared to the questions in Table I.
Get Compliance-Ready
Companies must have the appropriate plans and resources to comply with FSMA and certifications or face possible violations that can include fines and penalties under FDA enforcement. The questions in Table I will help companies identify areas to consider for Inspection readiness. Kestrel can also help answer questions, provide input on solutions, discuss how to better manage all of your food safety requirements—and change “No” responses into “Yes” responses that promote best practices for FSMA and food safety compliance.








